Theoretical properties of Thiazepine and its derivatives on inhibition of Aluminium Al (110) surface.
DOI:
https://doi.org/10.57056/ajet.v8i1.89Keywords:
DFT, Corrosion Inhibition, Physisorption, Al (110) surface, B3LYPAbstract
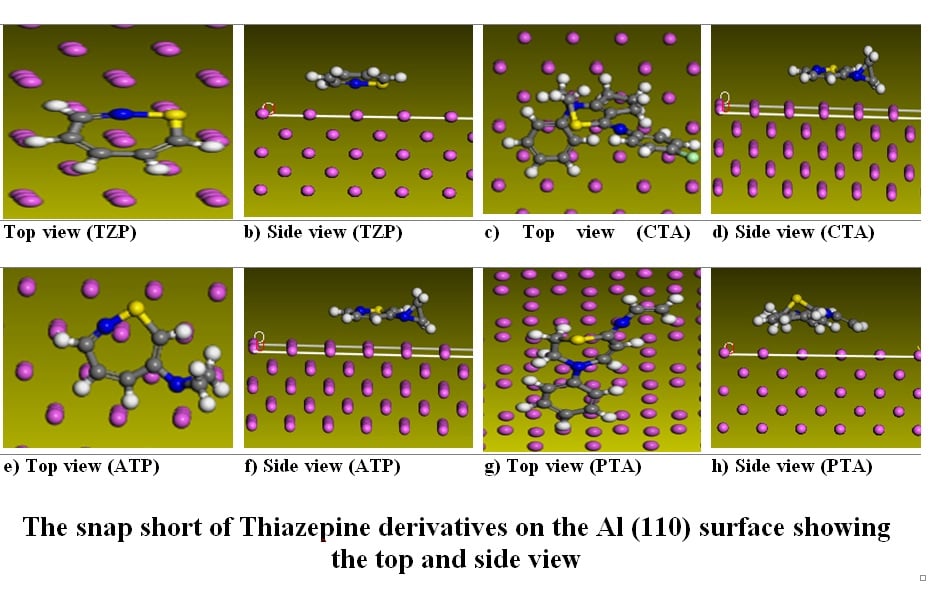
In this work, corrosion inhibition of Aluminium was evaluated theoretically through quantum functions. The studied molecules thiazepine and its derivatives were optimized and simulated with local density function B3LYP using DFT-D under restricted spin polarization DNP basis to obtain the stable geometry of the thiazepine structures. the Fukui density functions were evaluated to determine the frontier molecular orbitals (FMO) of the compounds. The number of adsorption sites (physisorption) was the mode of interaction with the heteroatoms such as Chlorine, Nitrogen, Sulphur oxygen and methylene (-CH2-) functional groups serving as the focal point for the selectivity of the donation and acceptance of electrons between the metal and the pyrimidine molecules as (ω+) electron accepting power and (ω−) electron donating power complement each other. The adsorption efficiency of the molecules as obtained by the simulated molecules was in the order PTA>CTA>ATP>TZP. Selectivity of the molecules was observed as the thiazepine molecules donate electrons more to p-orbital of the Al (110) surface.
References
Ayuba AM, Aminullahi A. Investigating the corrosion inhibition of strichnos spinosa L, extract on aluminium in 0.9M hydrochloric acid solution. Alger. J. Eng. Technol. 2020; 3: 28–37.
Umaru U, Ayuba A.M. Quantum chemical calculations and molecular dynamic simulation studies on the corrosion inhibition of aluminium metal by myricetin derivatives. Journal of new technology and materials 2020;10 (2):18-28.
Njoku DI, Onuoha GN, Oguzie EE, Oguzie KL, Egbedina AA, Alshawabkeh AN. Nicotiana tabacum leaf extract protects Aluminium alloy AA3003 from acid attack. Arab.J.Chem. https://doi.org/10.1016/j.arabjc.2016.07.017
Maryer TF, Christoph G, Christoph D. 16- Corrosion monitoring in concrete. Woodhead publishing seriesmin metals and surface engineering. Doi.org/10.10161b978-0-08-103003-5-00016-3 2021; 379-405.
Ayuba AM, Uzairu A, Abba H, Shallangwa G. A. Theoretical study of aspartic and glutamic acids as corrosion inhibitors on aluminium metal surface, J. Mater. Environ. Sci. 2018; 9:3026-3034.
Lgaz. H, Salghi R, Chaouiki S, Shubhalaxmi S, Jodeh K. S, Pyrazoline derivatives as possible corrosion inhibitors for mild steel in acidic media: A combined experimental and computational approach. Cogent Engineering. 2018; 5: 1441585.
Jyothi S, and Rathidevi K. Experimental and theoretical investigation oncorrosion inhibition of mild steel in sulphuricacid by coccinia indica leaves extract. RJ.Chem. 2017;10( 4):1253-1260
Joseph S, and John J. Electrochemical, quantum chemical, and molecular dynamic studies on the interaction of 4-amino-4H, 3,5-di(methoxy)-1,2,4-triazole (ATD), BATD, and DBATD on copper metal in 1N H2SO4. Materials and Corrosion. 2013;647:625-632.
Eddy NO, Ameh PO, Essien NB. Experimental and computational chemistry studies on the inhibition. Taibah University for science. 2018;12(5):545-556.
Ayuba AM, Aminullahi, A. Investigating the corrosion inhibition of strichnos spinosa L, extract on aluminium in 0.3M hydrochloric acid solution. Journal of applied science and environmental studies. 2020; 4(1):336-348.
Ayuba AM, Nyijime TA. Theoretical Study Of 2-Methyl Benzoazole and Its Derivatives As Corrosion Inhibitors On Aluminium. Metal Surface. J. Appl. Sci. Envir. Stud. 2021;4 (2):393-405
Smith SJ, Sutcliffe BT. The development of computational chemistry in the United Kingdom. Review in computational chemistry. 1972. 10:271-316.
El-Hendawy M M, Kamel AM, Mohamed MMA., Boukherroub R, Ryl, J, and Amin, MA. Diaryl sulphide derivatives as potential iron corrosion inhibitors: A Computational Study. Molecules. 2021; 26:01-12. https://doi.org/10.3390/molecules26206312.
Ayuba AM, Uzairu A, Abba H, Shallangwa GA. Hydroxycarboxylic acids as corrosion inhibitors on aluminium metal: Computational Study. Journal of Materials and Environmental Sciences. 2018;9(11): 3026-3034.
Muhammad S. and Ibrahim MB. Corrosion inhibition of zinc in 0.5 M HNO3 using Azadirchta Indicaca extract: experimental and computational study. Bayero Journal of Pure and Applied Science. 2022: 13(1):32-45. http://dx.doi.org/10.4314/bajopas.v13i1.1S
Usman B, Jimoh I, Bello AU. Theoretical study of 2-(3,4dihydroxyphenyl) croman-3,5,7-triol on corrosion inhibition of mild steel in acidic medium. Applied journal of environmental engineeering scienc. 2019;5 (1):66-74
Usman B, Hasmerya M, Hassan HA, Madzlan A. Computational evaluation of the effect of structural parameters of 3-flouro thiophene and 3-thiophene malonic acid on corrosion inhibition efficiency of mild steelin acid media. International journal of electromical science. 2015;10:3223-3229
Li WZ, Zhang Y, Zhai L, Ruan W, Zhang LW. Corrosion Inhibition of N80 Steel by Newly Synthesized Imidazoline Based Ionic Liquid in 15% HCl Medium: Experimental and Theoretical Investigations. International Journal of Electrochemical Science. 2020; 15: 722–739
Lgaz H, Saha A, Chaouiki KS, Bhat R Salghi R, Shubhalaxmi P, Banerjee IH, Ali MI, Khan, I. Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Construction and Building Materials. 2020; 233: 117320.
Kohn W, Becke AD and Parr RG. Density functional theory of electronic structure. The journal of physical chemistry. 1996. 100,31,12974-12980.
Ayuba AM, Nyijime TA, Muhammad AS. Density functional theory and molecular dynamic simulation studies on the corrosion inhibition of some Thiosemicarbazide derivatives on Aluminum metal. Journal of Applied Surfaces and Interface. 2020;8: 7-14
Ayuba AM, Uzairu A, Abba H, Shallangwa GA. Theoretical study of aspartic and glutamic acids as corrosion inhibitors on alumminium metal surface. Mor. J. Chem. 2018; 6(1): 160-172
Iorhuna F, Adulfatah SM, Ayuba AM. Quinazoline Derivatives as Corrosion Inhibitors on Aluminium Metal Surface: A Theoretical Study. Advanced Journal of Chemistry-Section A, 2023;6(1):71-84
Usman B, Marrof H, Abdallah HH, Aziz M, Jamaludin R, Alfakih AM. Corrosion inhibition efficiency of thiophene derivatives on mild steel: A QSAR model, International Journalof Electrochemical Science. 2014;.9: 1678-1689.
Ayuba AM, and Umar U. Modeling Vitexin and Isovitexin Flavones as Corrosion Inhibitors for Aluminium Metal. Karbala International Journal of Modern Science. 2021; (20): 30-45. https://doi.org/10.33640/2405-609X.31191e10.
Belghi ME, Dafali A, Karzaz Y, Bakasse M, Elalaui-Elalaui H Olasunkanmi LO, Ebenso E.E. Computational simumulation and statistical analysis on the relationship between corrosion inhibition efficiency and moleculat structure of some hydrazine derivatives in 2019; 491:707-722. phosphoric acid on mild steel surface. Applied surface science. http://doi.org/10.1016/j.apsus.2019.04.125
Ghassab M, Al-Mazaideh T, Ababneh S, Khalid HA, Rasheed MA. Jamhour Q, Haya J, Ayaal Salman, Ashraf M, Al-Msiedeen and Salim M. Khalil. DFT Calculations of Mesembryanthemum nodiflorum Compounds as Corrosion Inhibitors of Aluminum. Physical Science International Journal. 2016; 12(1): 1-7.
Ayuba AM, Abubakar. Inhibiting Aluminium acid corrosion using leaves extract of guiera senegalensis. J Fundam Appl Sci. 2021;13(2); 634-656. hpp/dx.doi.org/10.4314/jfas.v13i2.1.
Nyijime TA, and Iorhuna F. Theoretical study of 3-(4-hydroxyphenyl)-1-(4-nitrophenyl) prop-2-en-1-one and 3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one as corrosion inhibitors on mild steel. Appl. J. Envir. Eng. Sci. 2022; 8(2): 177-186
Glossman-Mitnik D, Computational Study of the Chemical Reactivity Properties of the Rhodamine B Molecule International Conference on Computational Science, ICCS Procedia Computer Science. 2013;18:816 – 825
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Fater Iorhuna, Nyime Aondofa Thomas, Saifullah Muhammad Lawal

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





