Adsorptive properties of 4-Hexyl-tertrahydro-thiopyran-1,1-diode on Al(110) and Fe(111) surface using DFT method
DOI:
https://doi.org/10.57056/ajet.v8i2.138Keywords:
DFT, Corrosion Inhibition, Al (110) surface, Fe(111) surface, Chemisorption, B3LYP, 4-Hexyl-Tertrahydro-Thiopyran-1,1-DiodeAbstract
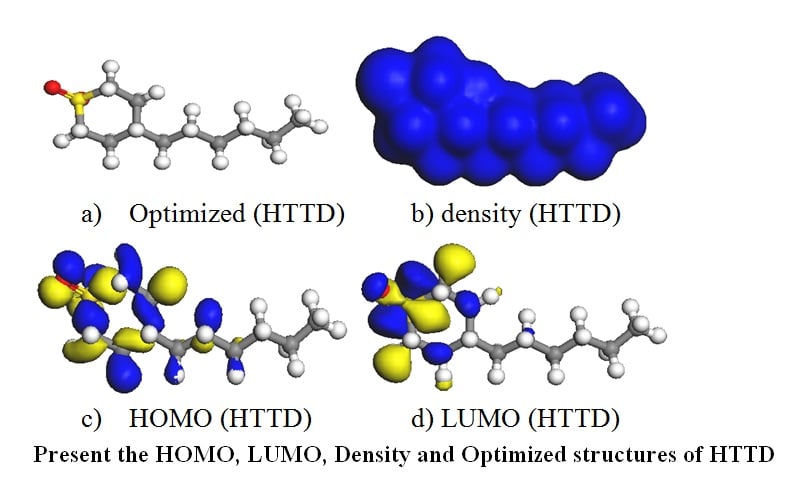
Aluminum and iron are highly significant industrial commodities that are used to make anything from tools and concrete construction to roofing sheets and other roofing-related products. Even though they generate a protective oxygen barrier that prevents corrosion, they are nevertheless susceptible to corrosion in extreme conditions. In order to achieve the stable shape of the molecule HTTD, a theoretical investigation on the corrosion inhibition of metals like Aluminum and iron was conducted utilizing local density function B3LYP under limited spin polarization DNP foundation. The values of local/global reactivity parameters, including the (ω+) electroaccepting power and (ω-) electrodonating power between the inhibitor molecule and the metal surfaces, were demonstrated. These parameters included EHOMO, ELUMO, energy gap (∆Eg), electronegativity (χ), global hardness (η), global electrophilicity index (ω), and fraction of electron transfer (∆N). Physisorption mode was defined as the mode of interaction of HTTD on Aluminium and Chemical Adsorption on Iron surface based on the simulation modeling output. The outcome of Fukui functions revealed that the focus point for the selectivity of electron donation and acceptance between the metal and the moiety is a hetero-atom present in the molecule such as oxygen, sulfur and the methylene (-CH2-) functional group.
References
Nyijime TA, Chahul HF, Ayuba AM. Iorhuna F. Theoretical Investigations on Thiadiazole Derivatives as Corrosion Inhibitors on Mild Steel. Adv. J. Chem. A. 2023;6(2):141-154. DOI: 10.22034/AJCA.2023.383496.1352
Umaru U, Ayuba A.M. Quantum chemical calculations and molecular dynamic simulation studies on the corrosion inhibition of aluminium metal by myricetin derivatives. Journal of new technology and materials 2020;10 (2):18-28
Kohn W, Becke AD and Parr RG. Density functional theory of electronic structure. The journal of physical chemistry. 1996; 100(31):12974-12980.
Ayuba AM, Nyijime TA, Muhammad AS. Density functional theory and molecular dynamic simulation studies on the corrosion inhibition of some Thiosemicarbazide derivatives on Aluminum metal. Journal of Applied Surfaces and Interface. 2020; 8: 7-14
Maryer TF, Christoph G, Christoph D. 16- Corrosion monitoring in concrete. Woodhead publishing seriesmin metals and surface engineering. 2021; 379-405. Doi.org/10.10161b978-0-08-103003-5-00016-3
Ayuba AM, Uzairu A, Abba H, Shallangwa G. A. Theoretical study of aspartic and glutamic acids as corrosion inhibitors on aluminium metal surface, J. Mater. Environ. Sci. 2018; 9:3026-3034
Jyothi S, and Rathidevi K. Experimental and theoretical investigation oncorrosion inhibition of mild steel in sulphuricacid by coccinia indica leaves extract. RJ.Chem. 2017;10(4):1253-1260
Joseph S, and John J. Electrochemical, quantum chemical, and molecular dynamic studies on the interaction of 4-amino-4H, 3,5-di(methoxy)-1,2,4-triazole (ATD), BATD, and DBATD on copper metal in 1N H2SO4. Materials and Corrosion. 2013;647:625-632.
Ayuba AM, Aminullahi, A. Investigating the corrosion inhibition of strichnos spinosa L, extract on aluminium in 0.3M hydrochloric acid solution. Journal of applied science and environmental studies. 2020; 4(1):336-348.
Ayuba AM, Nyijime TA. Theoretical Study of 2-methyl Benzoazole and its derivatives as corrosion inhibitors on Aluminium. Metal Surface. J. Appl. Sci. Envir. Stud. 2021;4 (2):393-405
Smith SJ, Sutcliffe BT. The development of computational chemistry in the United Kingdom. Review in computational chemistry. 1972. 10:271-316.
Ayuba AM, Uzairu A, Abba H, Shallangwa GA. Hydroxycarboxylic acids as corrosion inhibitors on Aluminium metal: Computational Study. Journal of Materials and Environmental Sciences. 2018;9(11): 3026-3034.
Li WZ, Zhang Y, Zhai L, Ruan W, Zhang LW. Corrosion inhibition of N80 steel by newly synthesized imidazoline based ionic liquid in 15% HCl medium: experimental and theoretical investigations. International Journal of Electrochemical Science. 2020; 15: 722–739
Lgaz H, Saha A, Chaouiki KS, Bhat R Salghi R, Shubhalaxmi P, Banerjee IH, Ali MI, Khan, I. Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid. Construction and Building Materials. 2020; 233: 117320.
Lgaz. H, Salghi R, Chaouiki S, Shubhalaxmi S, Jodeh K. S, Pyrazoline derivatives as possible corrosion inhibitors for mild steel in acidic media: A combined experimental and computational approach. Cogent Engineering. 2018; 5: 1441585.
Ayuba AM, Uzairu A, Abba H, Shallangwa GA. Theoretical study of aspartic and glutamic acids as corrosion inhibitors on alumminium metal surface. Mor. J. Chem. 2018; 6(1): 160-172
Usman B, Marrof H, Abdallah HH, Aziz M, Jamaludin R, Alfakih AM. Corrosion inhibition efficiency of thiophene derivatives on mild steel: A QSAR model, International Journalof Electrochemical Science. 2014;.9: 1678-1689.
Ayuba AM, and Umar U. Modeling Vitexin and Isovitexin Flavones as Corrosion Inhibitors for Aluminium Metal. Karbala International Journal of Modern Science. 2021; (20): 30-45. https://doi.org/10.33640/2405-609X.31191e10.
Belghi ME, Dafali A, Karzaz Y, Bakasse M, Elalaui-Elalaui H Olasunkanmi LO, Ebenso E.E. Computational simumulation and statistical analysis on the relationship between corrosion inhibition efficiency and moleculat structure of some hydrazine derivatives in 2019; 491:707-722. phosphoric acid on mild steel surface. Applied surface science. http://doi.org/10.1016/j.apsus.2019.04.125
Ghassab M, Al-Mazaideh T, Ababneh S, Khalid HA, Rasheed MA. Jamhour Q, Haya J, Ayaal Salman, Ashraf M, Al-Msiedeen and Salim M. Khalil. DFT calculations of mesembryanthemum nodiflorum compounds as corrosion inhibitors of Aluminum. Physical Science International Journal. 2016; 12(1): 1-7.
Nyijime TA, and Iorhuna F. Theoretical study of 3-(4-hydroxyphenyl)-1-(4-nitrophenyl) prop-2-en-1-one and 3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one as corrosion inhibitors on mild steel. Appl. J. Envir. Eng. Sci. 2022; 8(2): 177-186
Glossman-Mitnik D. Computational study of the chemical reactivity properties of the Rhodamine B Molecule International Conference on Computational Science, ICCS Procedia Computer Science. 2013;18:816 – 825
Iorhuna F, Adulfatah SM, Ayuba AM. Quinazoline derivatives as corrosion inhibitors on aluminium metal surface: A theoretical study. Advanced Journal of Chemistry-Section A, 2023;6(1):71-84
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Fater Iorhuna, Abdullahi Muhammad Ayuba , Safiyya Abubakar Minjibir, Thomas Aondofa Nyijime, Nura Ishaq

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





