Adsorptive, kinetic and thermodynamic investigations of Sarcocephalus latifolius leaves extract as corrosion inhibitor on alloy steel in 0.6M HCl solution
DOI:
https://doi.org/10.57056/ajet.v7i1.15Keywords:
Alloy steel, Sarcocephalus latifolius, Pysisorption, Freundlich isotherm, First order kineticsAbstract
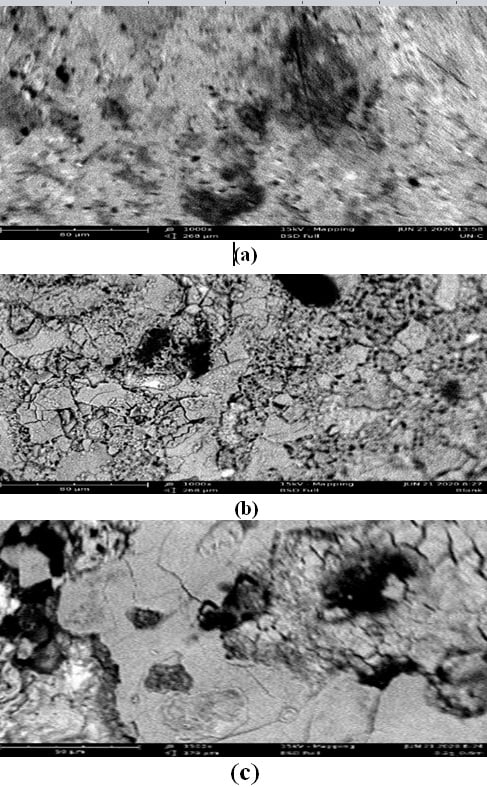
One of the materials that have many uses in the manufacturing, automotive, and construction industries is the alloy steel. When exposed to harsh environments this material is susceptible to deterioration and corrosion. Because of this reason, it is necessary to safeguard this valuable material. There have been many different methods used, but inhibition using particularly plant extract has reportedly been effective and acceptable to the environment. In this study, the behavior of alloy steel in 0.6M HCl at various concentrations of Sarcocephalus latifolius extract was assessed using weight loss and surface characterization methods of analysis. The plant extract's ability to inhibit corrosion increased during the weight loss experiment from 25.56% to 61.18% as concentration of the plant extract increased with a decrease in temperature from 323K to 303K respectively. Thermodynamic parameters of the inhibitory process were discovered to classify the process as feasible and spontaneous, obeying the Freundlich adsorption isotherm model's description of physical adsorption. The kinetic analysis of the inhibitory process demonstrates that it follows the first order model, with activation energy and half-life values rising with the concentration of the plant extract. In conclusion, Sarcocephalus latifolius extract is effective in inhibiting the corrosion of alloy steel in 0.6M HCl at low temperatures and higher plant extract concentrations through surface adsorption.
References
Abdullahi A, Ameenullah A. Corrosion inhibition potentials of Strichnos spinosa L. on Aluminium in 0.9 M HCl medium: experimental and theoretical investigations. Algerian Journal of Engineering and Technology. 2020; 3:28-37. http://dx.doi.org/10.5281/zenodo.4402204
Ayuba AM, Abdullateef A. Investigating the corrosion inhibition potentials of Strichnos spinosa L. Extract on aluminium in 0.3 M hydrochloric acid solution. Journal of Applied Science and Environmental Studies. 2021; 4(1):4-11. https://doi.org/10.48393/IMIST.PRSM/jases-v4i1.24275.
Popoola LT, Aderibigbe TA, Lala MA. Mild Steel Corrosion Inhibition in Hydrochloric Acid Using Cocoa Pod Husk-Ficus exasperata: Extract Preparation Optimization and Characterization. Iranian Journal of Chemistry and Chemical Engineering (IJCCE). 2022; 41(2):482-92. https://doi.org/10.30492/ijcce.2021.114540.3752
Beniken M, Driouch M, Sfaira M, Hammouti B, Ebn Touhami M, Mohsin M. Kinetic–thermodynamic properties of a polyacrylamide on corrosion inhibition for C-steel in 1.0 M HCl medium: part 2. Journal of Bio-and Tribo-Corrosion. 2018; 4(3):1-3. https://doi.org/10.1007/s40735-018-0152-1
Singh A, Ansari KR, Chauhan DS, Quraishi MA, Lgaz H, Chung IM. Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium. Journal of colloid and interface science. 2020; 560:225-236. https://doi.org/10.1016/j.jcis.2019.10.040
Zarrouk A, Zarrok H, Salghi R, Hammouti B, Bentiss F, Touir R, Bouachrine MO. Evaluation of N-containing organic compound as corrosion inhibitor for carbon steel in phosphoric acid. J Mater Environ Sci. 2013; 4(2):177-192.
Fazal BR, Becker T, Kinsella B, Lepkova K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. npj Materials Degradation. 2022; 6(1):1-4. https://doi.org/10.1038/s41529-021-00201-5
Li W, Zhang Z, Zhai Y, Ruan L, Zhang W, Wu L. Electrochemical and computational studies of proline and captopril as corrosion inhibitors on carbon steel in a phase change material solution. Int. J. Electrochem. Sci. 2020; 15(1):722-739. https://doi.org/10.20964/2020.01.63
Ekemini BI, Uwemedimo EU. Phytochemical profile, adsorptive and inhibitive of Costus afer extracts on aluminium corrosion in hydrochloric acid. Research Library Der Chemica Sinica, 2012, 3(6):1394-1405.
Rajamohan N, Al Shibli FS, Rajasimman M. Environmentally benign Prosopis juliflora extract for corrosion protection by sorption-Gravimetric, mechanistic and thermodynamic studies. Environmental Research. 2022; 203:111816. https://doi.org/10.1016/j.envres.2021.111816
Ayuba AM, Abubakar M. Inhibiting aluminium acid corrosion using leaves extract of Guiera senegalensis. Journal of Fundamental and Applied Sciences. 2021, 13(2):634-656. https://doi.org/10.4314/jfas.v13i2.1
Chahul HF, Ayuba AM, Nyior S. Adsorptive, Kinetic, Thermodynamic and Inhibitive Properties of Cissus Populnea Stem Extract on the Corrosion of Aluminum in Acid Medium. ChemSearch Journal. 2015; 6(1):20-30. http://dx.doi.org/10.4314/csj.v6i1.4
Abdullahi A, Muhammad A. DFT and molecular dynamic simulation study on the corrosion inhibition of Aluminum by some flavonoids of Guiera Senegalensis leaves. Algerian Journal of Engineering and Technology. 2021; 4:66-73. http://dx.doi.org/10.5281/zenodo.4636546
Husaini M, Usman B, Ibrahim MB. Study of corrosion inhibition performance of Glutaraldehyde on Aluminium in nitric acid solution. Algerian Journal of Engineering and Technology. 2020; 2:3-10 https://doi.org/10.5281/zendo.3923029
Wan S, Wei H, Quan R, Luo Z, Wang H, Liao B, Guo X. Soybean extract firstly used as a green corrosion inhibitor with high efficacy and yield for carbon steel in acidic medium. Industrial Crops and Products. 2022; 187:115354. https://doi.org/10.1016/j.indcrop.2022.115354
El Azzouzi M, Azzaoui K, Warad I, Hammouti B, Shityakov S, Sabbahi R, Saoiabi S, Youssoufi MH, Akartasse N, Jodeh S, Lamhamdi A. Moroccan, Mauritania, and senegalese gum Arabic variants as green corrosion inhibitors for mild steel in HCl: Weight loss, electrochemical, AFM and XPS studies. Journal of Molecular Liquids. 2022; 347:118354. https://doi.org/10.1016/j.molliq.2021.118354
Behera D, Nandi BK, Bhattacharya S. Variations in combustion properties of coal with average relative density and functional groups identified by FTIR analysis. International Journal of Coal Preparation and Utilization. 2022; 42(6):1695-1711. https://doi.org/10.1080/19392699.2020.1755661
Salman TA, Al-Azawi KF, Mohammed IM, Al-Baghdadi SB, Al-Amiery AA, Gaaz TS, Kadhum AA. Experimental studies on inhibition of mild steel corrosion by novel synthesized inhibitor complemented with quantum chemical calculations. Results in Physics. 2018; 10:291-296. https://doi.org/10.1016/j.rinp.2018.06.019
Eddy NO, Ameh PO, Essien NB. Experimental and computational chemistry studies on the inhibition of aluminium and mild steel in 0.1 M HCl by 3-nitrobenzoic acid. Journal of Taibah University for Science. 2018; 12(5):545-556. https://doi.org/10.1080/16583655.2018.1500514
de Vargas Brião G, da Silva MG, Vieira MG, Chu KH. Correlation of type II adsorption isotherms of water contaminants using modified BET equations. Colloid and Interface Science Communications. 2022; 46:100557. https://doi.org/10.1016/j.colcom.2021.100557
Umaru U, Ayuba AM. Quantum chemical calculations and molecular dynamic simulation studies on the corrosion inhibition of aluminium metal by myricetin derivatives. Journal of New Technology and Materials. 2020; 10:18-28.
Jyothi S, Rathidevi K. Experimental and theoretical investigation on corrosion inhibition of mild steel in sulphuric acid by coccinia indica leaves extract. Rasāyan Journal of Chemistry. 2017; 10(4):125312-60. http://dx.doi.org/10.7324/RJC.2017.1041924
Husaini M. Effect of Anisaldehyde as Corrosion Inhibitor for Aluminium in Sulphuric Acid Solution. Journal of Science and Technology. 2020; 12(2):1-10. https://doi.org/10.30880/jst. 2020.12.02.001
Ayuba AM, Uzairu A, Abba H, Shallangwa GA. Theoretical study of aspartic and glutamic acids as corrosion inhibitors on aluminium metal surface. Moroccan Journal of Chemistry. 2018; 6(1):160-172. https://doi.org/10.48317/IMIST.PRSM/morjchem-v6i1.9111
Hrimla M, Bahsis L, Boutouil A, Laamari MR, Julve M, Stiriba SE. A combined computational and experimental study on the mild steel corrosion inhibition in hydrochloric acid by new multifunctional phosphonic acid containing 1, 2, 3-triazoles. Journal of Adhesion Science and Technology. 2020; 34(16):1741-1773. https://doi.org/10.1080/01694243.2020.1728177.
Elmi S, Foroughi MM, Dehdab M, Shahidi-Zandi M. Computational evaluation of corrosion inhibition of four quinoline derivatives on carbon steel in aqueous phase. Iranian Journal of Chemistry and Chemical Engineering (IJCCE). 2019; 38(1):185-200.
Lgaz H, Saha SK, Chaouiki A, Bhat KS, Salghi R, Banerjee P, Ali IH, Khan MI, Chung IM. Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies. Construction and Building Materials. 2020; 233:117320. https://doi.org/10.1016/j.conbuildmat.2019.117320.
Dagdag O, Safi Z, Erramli H, Cherkaoui O, Wazzan N, Guo L, Verma C, Ebenso EE, El Harfi A. Adsorption and anticorrosive behavior of aromatic epoxy monomers on carbon steel corrosion in acidic solution: computational studies and sustained experimental studies. RSC advances. 2019; 9(26):14782-14796 https://doi.org/10.1039/C9RA01672D.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Fater Iorhuna, Ayuba Abdullahi

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





