Preliminary Phytochemical Screening, Quantification of phenolic compounds, of Plant Extract from Chenopodium quinoa
Main Article Content
Abstract
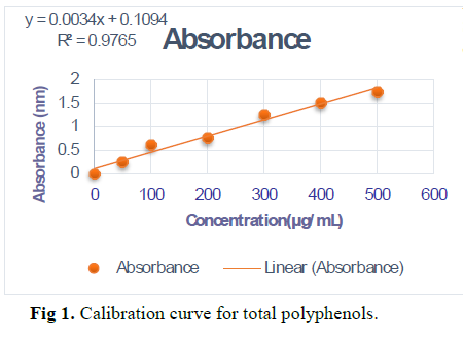
The aim of this study was to screening the phytogenic chemical compounds of the seeds of Chenopodium quinoa, obtained from Wilaya of El-oued. The chemical study showed that the plant contained a number of secondary metabolites, flavonoids, tannins, saponins, steroids and triterpenes, glycosids while the absence of alkaloids and comarin in Chenopodium quinoa. Folin-Ciocalteau colorimetric were used to determine the total phenolic con-tent (TPC), in the hydroalcoholic seeds extracts. The yield of the methanolic extract was estimated at 36.66%. As for the quantitative content of polyphenols, it is 11.647 ± 1.91µg AGE / mg extrait From this, Chenopodium quinoa is considered a nutritional and therapeutic value because it contains secondary metabolites
Article Details
References
Lindfors K, Ciacci C, Kurppa K, Lundin Knut E. A, Makharia Govind K, Luisa Mearin M, Murray Joseph A, Verdu Elena F. and Kaukinen Katri. Coeliac disease. Nature reviews Disease Primers. 2019, 5(1):3
DOI: 10.1038/s41572-018-0054-z DOI: https://doi.org/10.1038/s41572-018-0054-z
FAO. Quinoa: An ancient crop to contribute to world food security. Latin America and the Caribbean, 2011, pp: 3-14.
Capriles V D, Coelho K D, Guerra-Matías A C, and Arêas J A G. Effects of processing methods on amaranth starch
digestibility and predicted glycemic Index. Journal of Food Science. 2008 , 73:H160-H164. DOI: https://doi.org/10.1111/j.1750-3841.2008.00869.x
Bonifacio A. Chenopodium Sp.: Genetic resources, ethnobotany, and geographic distribution. Food Reviews International. 2003, 19: 1–7. DOI: https://doi.org/10.1081/FRI-120018863
Avila Ruiz G. Exploring novel food proteins and processing technologies A case study on quinoa protein and high pressure high temperature processing. Thesis submitted in fulfillment of the requirements for the degree of doctor. Wageningen
University. Wageningen. 2016 , p: 09.
Stikić R, Jovanović Z, Marjanović M, Đorđević S. The Effect of Drought on Water Regime and Growth of Quinoa
(Chenopodium quinoa Willd.). Research Grants: TR-31006 and EU FP7 project AREA. Serbia. 2015, 2: 52-8000
Harrar A, activités antioxydante et antimicrobienne d'extraits de Rhamnus alaternus L. Mémoire magister, Université Farhat Abbas (Sétif). 2012, 95p.
Falleh H, Ksouri R, Chaieb K, Karray-Bouraoui N, Trabelsi N, Boulaaba M, Abdelly C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. C. R. Biologies; 2008 , 331(5): 372-379 doi: 10.1016/j.crvi.2008.02.008 DOI: https://doi.org/10.1016/j.crvi.2008.02.008
Harborne J B. Phytochemical methods. A guide to modern techniques of plants analysis. Elsevier Science.1998 , 3 :11.
Rahman G, Syed U Jan, Syed F, Samiullah S, Nusrat J. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan; Hindawi e Scientific World Journal. 2017 , 1-7. https://doi.org/10.1155/2017/5873648 DOI: https://doi.org/10.1155/2017/5873648
Katiki L M, Chagas A C S, Takahira R K, Juliani H R, Ferreira J F S, Amarante A F T. Evaluation of Cymbopogon schoenanthus Essential Oil in Lambs Experimentally Infected With Haemonchus contortus. Veterinary parasitology. 2012 , 186(4): 312- 318. Https://Doi.Org/10.1016/J.Vetpar. 2011.12.003 DOI: https://doi.org/10.1016/j.vetpar.2011.12.003
Ayoola G A, Coker H A B, Adesegun S A, Adepoju-Bello A A, Obaweya K, EC Ezennia E C, Atangbayila T O.Phytochemical Screening and Antioxidant Activities of Some Selected Medicinal Plants Used for Malaria, Therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research. 2008 , 7 (3) : 1019-1024. DOI: https://doi.org/10.4314/tjpr.v7i3.14686
Kamal T, Ahmad M, Raji AA, Muayad S, Rahma , Muhammad N O, Preliminary phytochemical screening test of Garcinia griffithii Plant. Innova Ciencia. 2012 , 4(4): 68-74.
Ribéreau-Gayon P . Les composés phénoliques des végétaux. Editions Dunod, Paris. 1968 , 254 pp
López-Fernández O, Domínguez R, Pateiro M, Munekata Paulo E.S., Rocchetti G, and Lorenzo José M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review Antioxidants (Basel). 2020 , 9(6): 479. doi: 10.3390/antiox9060479 DOI: https://doi.org/10.3390/antiox9060479
Singleton V L, Orthofer R, Lamuela-Raventos R M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants By Means of Folin- Ciocalteu Reagent Methode. Enzymologie . 1999 , 299: 152-178 Http://Dx.Doi.Org/10.1016/S0076-6879(99)99017-1. DOI: https://doi.org/10.1016/S0076-6879(99)99017-1
Slinkard K, Singleton V L. Total Phenol Analys: Automation and Comparison With manual Methods. American journal of enology and viticulture. 1977 , 28: 49-55. DOI: https://doi.org/10.5344/ajev.1974.28.1.49
Gordillo-Bastidas E, Díaz-Rizzolo DA, Roura E, Massanés T, Gomis R. - Quinoa (Chenopodium quinoa Willd). From
Nutritional Value to Potential Health Benefits: An Integrative Review. J Nutr Food Sci. 2016, 6(3): 2155-9600 DOI: 10.4172/2155-9600.1000497 DOI: https://doi.org/10.4172/2155-9600.1000497
Jancurová M, Minarovičová L, Dandár A, - Quinoa – a review. Czech J. Food Sci. 2009, Vol. 27: 71–79. DOI: https://doi.org/10.17221/32/2008-CJFS
Maradini A, Pirozi M, Borges J, Sant'Ana H, Chaves J, Coimbra J, - Quinoa: Nutritional, functional and antinutritional aspects. Crit Rev Food Sci Nutr. 2017 , 24;57(8):1618-1630. doi: 10.1080/10408398.2014.1001811. DOI: https://doi.org/10.1080/10408398.2014.1001811