Influence of leaf extracts and total flavonoids of Rhus tripartita (Ucria) Grande on phytobeneficial bacteria associated with its rhizosphere
Main Article Content
Abstract
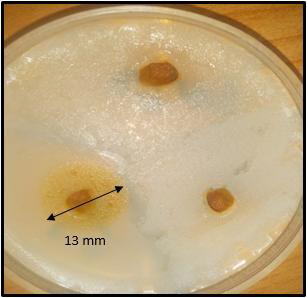
The article deals with the antimicrobial effect of Rhus tripartita (Ucria) Grande leaf extracts and total flavonoids against twelve antagonists Plant Growth Promoting Rhizobacteria of its rhizosphere, characterized in a previous study. The aim of this study is to demonstrate that leaves through their decomposition in the soil, may affect the distribution of bacterial communities in the rhizosphere. Leaves extracts were performed with distilled water, alcohol, methanol, hexane and chloroform as solvent and diluted in concentrations of 0.001, 0.01 and 0.1 mg/mL. The extraction of total flavonoids was carried out from leaves’ methanolic extract. The antimicrobial effect of the extracts was evaluated by the agar diffusion method and the determination of the minimum inhibitory concentration was carried out on a liquid medium. Alcohol, chloroform and methanol extracts were found to be the most effective on tested strains. The maximum zone inhibition was 18 mm, and the minimum zone inhibition was 7 mm. Rt 1: Bacillus licheniformis appears to be the most sensitive to all extracts. In contrast, Rt 7: Bacillus megaterium, seems to be the less sensitive strain. On the other hand, total flavonoids had a significant effect on 25% of the strains tested, mainly Bacillus genus. With a broad antimicrobial spectrum, the Rhus tripartita leaf extracts can be considered as a control agent for the distribution of the bacterial community in the rhizosphere. Therefore, our work showed that the plant could influence the bacterial diversity of its rhizosphere through its leaves.
Article Details
References
Abbassi F., Hani K. In vitro antibacterial and antifungal activities of Rhus tripartitum used as antidiarrhoeal in Tunisian folk medicine, Nat Prod Res. 2011; 26 (23): 2215-2218. https://doi.org/10.1080/14786419.2011.639072 DOI: https://doi.org/10.1080/14786419.2011.639072
Akroum S., Bendjeddou D., Satta D., Lalaoui K. Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia, Ame-Eur J Sci Res. 2009; 4(2): 93-96. DOI: https://doi.org/10.4103/0973-8258.69174
Alapetite G.P. Flore de la Tunisie. Angiopermes- Dicotyledones. Gamopetales. Tunis: Ministère de l'Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l'Agriculture. 1981 : 655-1190.
Ammar S., Mahjoub M.A., Mighri Z. Isolement pour la première fois et études structurales d’acides gras, de l’acide shikimique et d’un héteroside flavonique à partir de la plante Rhus tripartitum, J.Soc. Chim. Tun. 2004 ; 6 : 9-16.
Bais H.P., Weir T.L., Perry L.G., Gilroy S., Vivanco J.M. The role of root exudates in rhizosphere interactions with plants and other organisms, Annu. Rev. Plant Biol. 2006; 57: 233-266. https://doi.org/10.1146/annurev.arplant.57.032905.105159 DOI: https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bais H.P., Park S.W., Weir T.L., Callaway R.M., Vivanco, J.M. How plants communicate using the underground information super highway, Tren pl sci. 2004; 9(1): 26-32. https://doi.org/10.1016/j.tplants.2003.11.008 DOI: https://doi.org/10.1016/j.tplants.2003.11.008
Bais H.P., Loyola-Vargas V.M., Flores H.E., Vivanco J. M. Root-specific metabolism: the biology and biochemistry of underground organs, In Vitro Cellular & Developmental Biology-Plant. 2001; 37(6): 730-741.
https://doi.org/10.1007/s11627-001-0122-y DOI: https://doi.org/10.1007/s11627-001-0122-y
Barka Z.B., Aouadhi C., Tlili M., Alimi H., Miled H.B., Rhouma K.B., et al. Evaluation of the anti-diarrheal activity of the hydromethanolic root extract of Rhus tripartita (Ucria)(Anacardiacae), Biomedicine & Pharmacotherapy. 2016; 3: 827-834.
https://doi.org/10.1016/j.biopha.2016.07.055 DOI: https://doi.org/10.1016/j.biopha.2016.07.055
Benaissa A., Djebbar R., Abderrahmani A. Antagonistic effect of plant growth promoting rhizobacteria associated with Rhus tripartitus on Gram positive and negative bacteria. Analele Universităţii din Oradea, Fascicula Biologie. 2019; 26(2): 67- 72
Cushnie T.T., Hamilton V.E., Lamb A.J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports, Micro res. 2003; 158(4): 281-289. https://doi.org/10.1078/0944-5013-00206 DOI: https://doi.org/10.1078/0944-5013-00206
Delarras C. Microbiologie. 90 heures de travaux pratiques : eneignement commun et préparation à génie de l’environnement. Gaétan Morien Editeur. Europe. 1998. ISBN: 291074907X, 9782910749071; 169-178p.
Dewanto V., Wu X., Adom K. K., Liu R. H. Thermal 374 processing enhances the nutritional value of tomatoes by increasing 375 total antioxidant activity, J. Agri. Food. Chem. 2002; 50: 3010-3014. https://doi.org/10.1021/jf0115589 DOI: https://doi.org/10.1021/jf0115589
Giller K.E., Beareb M.H., Lavellec P., Izacd M.N., Swifte M.J. Agricultural intensification, soil biodiversity and agroecosystem function, App Soi Eco. 1997; 6 (1): 3-16. https://doi.org/10.1016/S0929-1393(96)00149-7 DOI: https://doi.org/10.1016/S0929-1393(96)00149-7
Gurusiddaiah S., Winward L.D., Burger D., Graham S.O. Pantomycin: a new antimicrobial antibiotic- Mycologia. 1979; 71(1): 103-118. https://doi.org/10.1080/00275514.1979.12020991 DOI: https://doi.org/10.1080/00275514.1979.12020991
Habibi A.A., Zubek S.A., Elbaz A., El-Khodery S.A., Osman H.Y., Bennour E.M.. Evaluation of Antibacterial Activity of Selected Libyan Medicinal Plants against Staphylococcus aureus and Bacillus subtilis. J Pharma Phytochem. 2015; 4(3): 16-20.
Habibi A.A., Zubek S.A., Abushhiwa M.A., Ahmed M.O., El- Khodery S.A., Osman H.Y., Bennour E.M. Antibacterial activityof selected Libyan medicinal plants against Pseudomonas aeruginosa and Escherichia coli, J Pharma and Phytochem. 2015;3(6): 197-201.
Holl F.B., Chanway C.P. Rhizosphere colonization and seedling growth promotion of lodgepole pine by Bacillus polymyxa, Can. J. Microbiol. 1992; 38: 303-308. https://doi.org/10.1139/m92-050 DOI: https://doi.org/10.1139/m92-050
Kang J., Li Z., Wu T., Jensen G.S., Schauss A.G., Wu X. Anti- oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.), Food Chem. 2010; 122(3): 610-617. https://doi.org/10.1016/j.foodchem.2010.03.020 DOI: https://doi.org/10.1016/j.foodchem.2010.03.020
Kümmerer K. Resistance in the environment, J Antimicro Chemother. 2004; 54(2): 311-320.
https://doi.org/10.1093/jac/dkh325 DOI: https://doi.org/10.1093/jac/dkh325
Lamb E.G., Kennedy N., Siciliano S.D. Effects of plant species richness and evenness on soil microbial community diversity and function, Pl. Soil. 2011; 338(1-2): 483-495. https://doi.org/10.1007/s11104-010-0560-6 DOI: https://doi.org/10.1007/s11104-010-0560-6
Liang J.G., Tao R.X., Hao Z.N., Wang L.P., Zhang X. Induction of resistance in cucumber against seedling damping-off by plant growth-promoting rhizobacteria (PGPR) Bacillus megaterium strain L8, Afr. J. Biotech. 2011; 10(36): 6920-6927. DOI: 10.5897/AJB11.260
Mahjoub M.A., Ammar S., Chatter R., Abbassi F., Hani K., Bouraoui A., Mighri Z. Contribution to the biological and the chemical study of Rhus tripartitum growing in Tunisia, Rev Reg Arid. 2007; 441-448.
Mahjoub M.A, Ammar S., Edziri H., Mighri N., Bouraoui A., Mighri Z. Anti-inflammatory and antioxidant activities of some extracts and pure natural products isolated from Rhus tripartitum (Ucria), Med Chem Res. 2010; 19(3): 271-282.
https://doi.org/10.1007/s00044-009-9190-z DOI: https://doi.org/10.1007/s00044-009-9190-z
Mandalari G., Bennett R. N., Bisignano G., Trombetta D., Saija A., Faulds C. B., & Narbad A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry, Journal of Applied Microbiology. 2007; 103(6): 2056-2064. https://doi.org/10.1111/j.1365-2672.2007.03456.x DOI: https://doi.org/10.1111/j.1365-2672.2007.03456.x
Martini N. D., Katerere D. R., Eloff J. N., Jager A. K., Van Staden J. Seven flavonoids with antibacterial activity isolated from Combretum erythrophyllum, South Afr J Bot. 2004; 70(2): 310-312. DOI: https://doi.org/10.1016/S0254-6299(15)30251-9
Mishra P.K., Joshi P., Suyal P., Bisht J.K., Bhatt J.C. Potential of Phosphate Solubilising Microorganisms in Crop Production. In: Chandra R., Raverkar K.P. Bioresources of sustainable plant nutrient management. India: 2014, 201-212.
Mohammed A.E.S.I. Phytoconstituents and the study of antioxidant, antimalarial and antimicrobial activities of Rhus tripartita growing in Egypt, J Pharma Phytochem. 2015; 4(2): 276-281.
Nadeem S.M., Shaharoona B., Arshad M., Crowley D.E. Population density and functional diversity of plant growth promoting rhizobacteria associated with avocado trees in saline soils, App. Soi. Eco. 2012; 62: 147-154. https://doi.org/10.1016/j.apsoil.2012.08.005 DOI: https://doi.org/10.1016/j.apsoil.2012.08.005
Nardi S., Concheri G., Pizzeghello D., Sturaro A., Rellac R., Parvoli G. Soil organic matter mobilization by root exudates, Chemosphere. 2000; 41(5): 653-658. https://doi.org/10.1016/S0045-6535(99)00488-9 DOI: https://doi.org/10.1016/S0045-6535(99)00488-9
Rauha J. P., Remes S., Heinonen M., Hopia A., Kähkönen M., Kujal, T., Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds, International journal of food microbiology. 2000; 56(1): 3-12. DOI: https://doi.org/10.1016/S0168-1605(00)00218-X
https://doi.org/10.1016/S01681605(00)00218-X
Sen A., Batna A. Evaluation of antimicrobial activity of different solvent extracts of medicinal plant : Melia azedarach L. Int J Curr Pharm Res. 2012; 4(2): 67-73.
Sohn H.Y., Son K.H., Know C.S., Kang S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants : Mors albal., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedecine. 2004, 11(7-8): 666-672. DOI: https://doi.org/10.1016/j.phymed.2003.09.005 DOI: https://doi.org/10.1016/j.phymed.2003.09.005
Susilowate D.N., Sudiana I.M., Mubarik N.K., Swanto A. Species and functional diversity of rhizobacteria of rice plant in the coastal soils of Indonisia. Int J Agr Sci. 2015; 16(1): 39-50. http://repository.pertanian.go.id/handle/123456789/116 DOI: https://doi.org/10.21082/ijas.v16n1.2015.39-50
Taguri T., Tanaka T., Kounou I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-bome disease. Biol Pharm Bull . 2004; 27(12): 1965-1969. DOI: 10.1248/bpb.27.1965 DOI: https://doi.org/10.1248/bpb.27.1965
Tlili N., Mejri H., Yahia Y., Saadaoui E., Rejeb S., Khaldi A., Nasri N. Phytochemicals and antioxidants of Rhus tripartitum (Ucria) fruits depeding on locality and different stages of maturity. Food chemistry. 2014; 160(2014):98-103.
DOI:10.1016/j.foodchem.2014.03.030 DOI: https://doi.org/10.1016/j.foodchem.2014.03.030
Walker V. Impact de l’inoculation de microorganismes phytobénéfiques sur le métabolisme secondaire de Zea mays L. Sciences agricoles. Université de Claude Bernard-Lyon I. France. 2010. https://tel.archives-ouvertes.fr/tel- 00881136/document.
Wayne, PA. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi,. Clinical and Laboratory Standards Institute (CLSI). 3rd ed. IL: Allured Publishing Corporation. United States; 2008.