Synthesis and biological activity of 2-((3-Cyano-4,6-distyrylpyridin-2-yl) thio) acetamide and its cyclized form
Main Article Content
Abstract
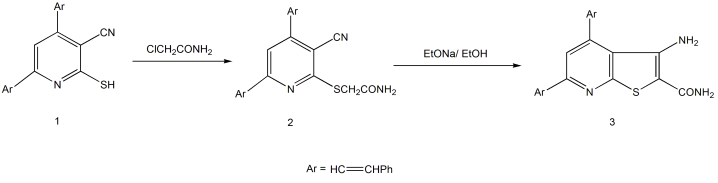
In this paper, 2-((3-Cyano-4,6-distyrylpyridin-2-yl)thio)acetamide (2) and its cyclized form, 3-amino-4,6-distyrylthieno[2,3-b]pyridine-2-carboxamide (3), were prepared and their structure characterizations were performed by the means of elemental and spectroscopic analyses. Their biological activity as insecticides against cowpea aphid Aphis craccivora Koch using acetamiprid insecticide as a reference was studied. The bioassay results for compounds (2) and (3) against nymphs of cowpea aphid showed that the LC50 values were 0.192 and 0.841 ppm, respectively, after 24 h of treatment but the LC50 values were 0.041 and 0.095 ppm, respectively, after 48 h of treatment. Furthermore, the bioassay results for compounds (2) and (3) showed that the LC50 values were 1.233 and 2.949 ppm, respectively, after 24 h of treatment and the LC50 values were 0.142 and 0.270 ppm, respectively, after 48 h of treatment against adults of cowpea aphid. Given these observations, it has been found that there is a remarkable relationship between the biological activity and the structure of the used compounds.
Article Details
References
Al-Taifi EA, Abdel-Raheem ShAA, Bakhite EA. Some reactions of 3-cyano-4-(p-methoxyphenyl)-5-oxo-5,6,7,8-tetrahydroquinoline-2(1H)-thione; Synthesis of new tetrahydroquinolines and tetrahydrothieno[2,3-b]quinolines. Assiut University Journal of Chemistry (AUJC). 2016, 45: 24-32. DOI: https://doi.org/10.21608/aunj.2016.221638
Kamal El-Dean AM, Abd-Ella AA, Hassanien R, El-Sayed MEA, Zaki RM, Abdel-Raheem ShAA. Chemical design and toxicity evaluation of new pyrimidothienotetrahydroisoquinolines as potential insecticidal agents. Toxicology Reports. 2019, 6: 100-104. DOI: https://doi.org/10.1016/j.toxrep.2018.12.004
Bakhite EA, Abd-Ella AA, El-Sayed MEA, Abdel-Raheem ShAA. Pyridine derivatives as insecticides. Part 1: Synthesis and toxicity of some pyridine derivatives against Cowpea Aphid, Aphis craccivora Koch (Homoptera: Aphididae). Journal of Agricultural and Food Chemistry. 2014, 62(41): 9982–9986. DOI: https://doi.org/10.1021/jf503992y
Bakhite EA, Abd-Ella AA, El-Sayed MEA, Abdel-Raheem ShAA. Pyridine derivatives as insecticides. Part 2: Synthesis of some piperidinium and morpholinium cyanopyridinethiolates and their Insecticidal Activity. Journal of Saudi Chemical Society. 2017, 21(1): 95–104. DOI: https://doi.org/10.1016/j.jscs.2016.02.005
Abdel-Raheem ShAA, Kamal El-Dean AM, Hassanien R, El-Sayed MEA, Abd-Ella AA. Synthesis and characterization of some distyryl-derivatives for agricultural uses. European Chemical Bulletin. 2021, 10(1): 35-38. DOI: https://doi.org/10.17628/ecb.2021.10.35-38
Altaf AA, Shahzad A, Gul Z, Rasool N, Badshah A, Lal B, Khan EA. Review on the Medicinal Importance of Pyridine Derivatives. Journal of Drug Design and Medicinal Chemistry. 2015, 1: 1-11. DOI: https://doi.org/10.1155/2015/913435
Kamal El-Dean AM, Abd-Ella AA, Hassanien R, El-Sayed MEA, Abdel-Raheem ShAA. Design, Synthesis, Characterization, and Insecticidal Bioefficacy Screening of Some New Pyridine Derivatives. ACS Omega. 2019, 4: 8406-8412. DOI: https://doi.org/10.1021/acsomega.9b00932
Zhang N, Tomizawa M, Casida JE. α-Nitro Ketone as an Electrophile and Nucleophile: Synthesis of 3-Substituted 2-Nitromethylenetetrahydrothiophene and tetrahydrofuran as Drosophila Nicotinic Receptor Probes. The Journal of Organic Chemistry. 2004, 69: 876-881. DOI: https://doi.org/10.1021/jo035457g
Shimomura M, Yokota M, Ihara M, Akamatsu M, Sattelle DB, Matsuda K. Role in the Selectivity of Neonicotinoids of Insect-Specific Basic Residues in Loop D of the Nicotinic Acetylcholine Receptor Agonist Binding Site. Molecular Pharmacology. 2006, 70: 1255–1263. DOI: https://doi.org/10.1124/mol.106.026815
Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annual Review of Entomology. 2003, 48: 339–364.
Tomizawa M, Talley T, Maltby D, Durkin KA, Medzihradszky KF, Burlingame AL, Taylor P, Casida JE. Mapping the elusive neonicotinoid binding site. Proceedings of the National Academy of Sciences of the United States of America. 2007, 104: 9075–9080. DOI: https://doi.org/10.1073/pnas.0703309104
Kagabu S, Ishihara R, Nishimura K, Naruse Y. Insecticidal and neuroblocking potencies of variants of the imidazolidine moiety of imidacloprid-related neonicotinoids and the relationship to partition coefficient and charge density on the pharmacophore. Journal of Agricultural and Food Chemistry. 2007, 55: 812–818. DOI: https://doi.org/10.1021/jf0623440
Yang ZB, Hu DY, Zeng S, Song BA. Novel hydrazone derivatives containing pyridine amide moiety: Design, synthesis, and insecticidal activity. Bioorganic & Medicinal Chemistry Letters. 2016, 26: 1161-1164. DOI: https://doi.org/10.1016/j.bmcl.2016.01.047
Tian Z, Shao X, Li Z, Qian X, Huang Q. Synthesis, insecticidal activity and QSAR of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification. Journal of Agricultural and Food Chemistry. 2007, 55: 2288−2292. DOI: https://doi.org/10.1021/jf063418a
Stivaktakis PD, Kavvalakis MP, Tzatzarakis MN, Alegakis AK, Panagiotakis MN, Fragkiadaki P, Vakonaki E, Ozcagli E, Hayes WA, Rakitskii VN, Tsatsakis AM. Long-term exposure of rabbits to imidaclorpid as quantified in blood induces genotoxic effect. Chemosphere. 2016, 149: 108-113. DOI: https://doi.org/10.1016/j.chemosphere.2016.01.040
Vardavas AI, Ozcagli E, Fragkiadaki P, Stivaktakis PD, Tzatzarakis MN, Kaloudis K, Tsardi M, Datseri G, Tsiaoussis J, Tsitsimpikou C, Carvalho F, Tsatsakis AM. DNA damage after long-term exposure of rabbits to Imidacloprid and sodium tungstate. Toxicology Letters. 2016, 258S: S247- S248. DOI: https://doi.org/10.1016/j.toxlet.2016.06.1878
Vardavas AI, Ozcagli E, Fragkiadaki P, Stivaktakis PD, Tzatzarakis MN, Alegakisa AK, Vasilakia F, Kaloudisa K, Tsiaoussisc J, Kouretasd D, Tsitsimpikoue Ch, Carvalhof F, Tsatsakis AM. The metabolism of imidacloprid by aldehyde oxidase contributes to its clastogenic effect in New Zealand rabbits. Mutation Research - Genetic Toxicology and Environmental Mutagenesis. 2018, 829–830: 26–32. DOI: https://doi.org/10.1016/j.mrgentox.2018.03.002
Stivaktakis P, Kavvalakis M, Goutzourelas N, Stagos D, Tzatzarakis M, Kyriakakis M, Rezaee R, Kouretas D, Hayes W,
Tsatsakis A. Evaluation of oxidative stress in long-term exposed rabbits to subtoxic levels of imidacloprid. Toxicology Letters. 2014, 229S, No. S228. DOI: https://doi.org/10.1016/j.toxlet.2014.06.764
O’Brien PJ, Abdel-Aal YA, Ottea JA, Graves JB. Relationship of insecticide resistance to carboxylesterases in Aphis gossypii (Homoptera: Aphididae) from Midsouth cotton. Journal of Economic Entomology. 1992, 85: 651–657. DOI: https://doi.org/10.1093/jee/85.3.651
Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925, 18: 265–267. DOI: https://doi.org/10.1093/jee/18.2.265a
Finney (Ed.) DJ. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve. Cambridge University Press, Cambridge, U. K. 1952.