Arbuscular Mycorrhiza as an Essential Ecotechnological Tool: A Critical Review of Literature on the Role of AMF in the Sustainability of Cultivation and Conservation of Palms
Main Article Content
Abstract
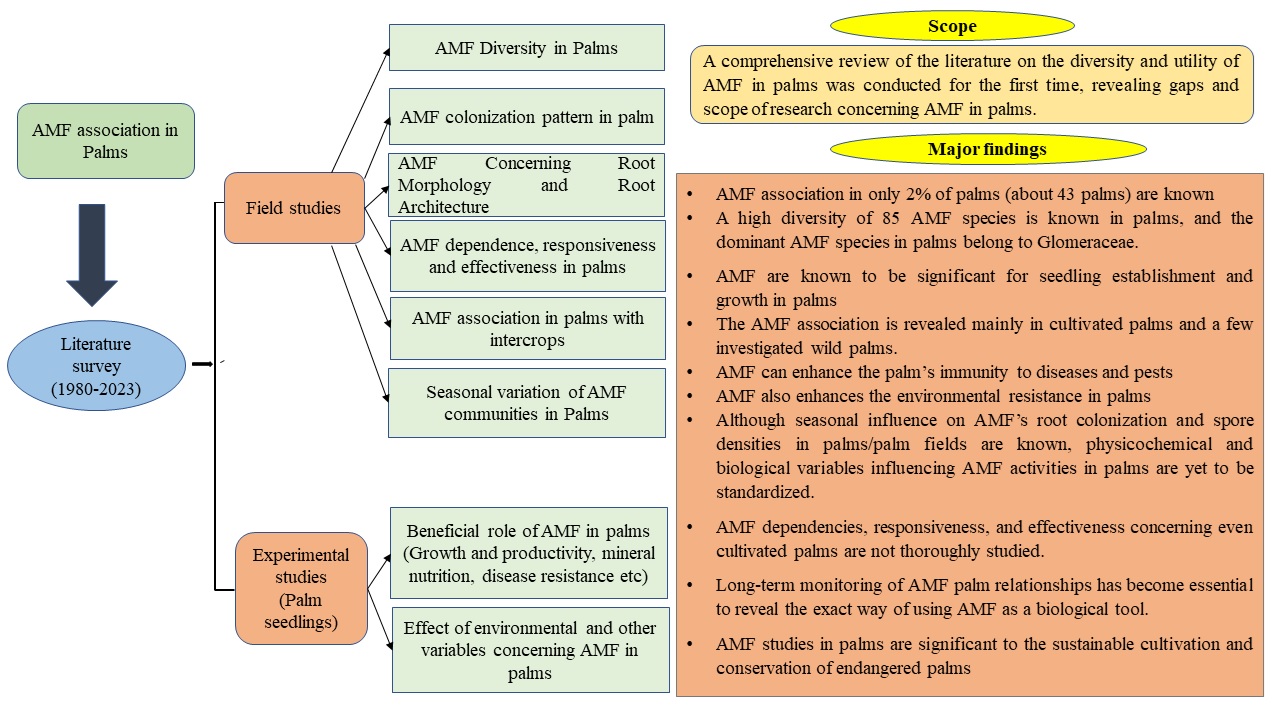
Palms are an ecologically and economically significant family of plants, including many crops. Sound knowledge of the ecology of arbuscular mycorrhizal fungal (AMF) association in plants is essential to the sustainable cultivation of crops and the conservation of sensitive species. The current Review is the first-ever comprehensive critical analysis of literature on AMF in the sustainable cultivation and conservation of palms, which reveals the gaps in existing studies and explains the specific needs of future investigations on AMF in Palms. AMF in only 2% of the known palms explored so far; a majority of wild palms and cultivated palms in many different regions remain unexplored. However, currently, a high diversity of 85 species of AMF from about 43 palms are known. The beneficial roles of AMF in palms include boosting productivity, assisting in the in-vitro raising of seedlings, and providing immunity to diseases and environmental stress. However, the identification of external and internal variables crucial to AMF association in palms in the field, long-term monitoring of AMF's beneficial influence in palms, and experimental application of AMF from wild palms in cultivated palms are further required. Overall, AMF dependence, responsiveness, and effectiveness in palms also need thorough investigation in the future.
Article Details
References
Plotkin, MJ.; Balick, MJ. Medicinal uses of South American palms. J Ethnopharmacol 1984, 10: 157–179.
Kissling, WD.; Balslev, H.; Baker, WJ.; Dransfield, J.; Göldel, B.; Lim, JY.; Onstein, RE.; Svenning, JC. PalmTraits 1.0, a species-level functional trait database of palms worldwide. Sci Data 2019, 6: 178. https://doi.org/10.1038/s41597-019-0189-0.
Almaaty, AHA.; Keshk, S.; Galal, A.; Abbas, OA.; Hassan, MK. Medicinal usage of some Arecaceae family members with potential anticancer effect. J Biotech Res 2022, 13: 55–63. https://www.proquest.com/scholarly-journals/medicinal-usage-some-arecaceae-family-members/docview/2649317822/se-2.
Baker, WJ.; Dransfield, J. Beyond Genera Palmarum: progress and prospects in palm systematics. Bot J Linn Soc 2016, 182: 207–233. https://doi.org/10.1111/boj.12401.
Renuka, C.; Sreekumar, VB. A field guide to the palms of India, Kerala Forest Research Institute, Peechi, India; 2012. http://www.kfri.org/.
Moore, EH.; Uhl, WN. Encyclopedia Britannica. https://www.britannica.com/plant/Arecales. Accessed on 28th October 2022.
Johnson, DV. Multi-purpose palms in agroforestry: A classification and assessment. Int Tree Crops J 1983, 2: 217–244. https://doi.org/10.1080/01435698.1983.9752757.
Kahn, F. Ecology of economically important Palms in Peruvian Amazonia. Adv Econ Bot 1988, 6: 42–49.
Johnson, DV. Tropical palms, Food and Agriculture Organization of the United Nations, Rome;1998.
Meerow, AW.; Krueger, RR.; Singh, R.; Low, E-TL.; Ithnin, M.; Ooi, LC-L. Coconut, Date, and Oil Palm Genomics. In Genomics of Tree Crops, Schnell, RJ., Priyadarshan, PM., Eds.; Springer, 2012; pp. 299-351. https://doi.org/10.1007/978-1-4614-0920-5.
Reichgelt, T.; West, CK.; Greenwood, DR. The relation between global palm distribution and climate. Sci Rep 2018, 8: 4721. https://doi.org/10.1038/s41598-018-23147-2.
Ehara, H.; Prathumyot, W.; Naito, H. Salt Resistance Mechanism of Metroxylon sagu, Starch-producing Palm. In Proceedings of the 7th ACSA Conference, IPB International Convention Center Bogor, Indonesia, 27-30 September 2011.
Hurtado, FHM.; Mosquera-Espinosa, AT.; Gomez-Carabali, A.; Otero, YJT. Temporal variation in arbuscular mycorrhizal fungi colonization of Bactris gasipaes Kunth in Buenaventura, Colombia. Acta Agron 2013, 62 (4): 344–35.
Dias, MMDS.; Noratto, G.; Martino, HSD.; Arbizu, S.; Peluzio, MdoCG.; Talcott, S.; Ramos, AM.; Mertens-Talcott, SU. Pro-Apoptotic Activities of Polyphenolics from Açai (Euterpe oleracea Martius) in Human SW-480 Colon Cancer Cells. Nutr Cancer 2014, 1–12. https://doi.org/10.1080/01635581.2014.956252.
Dias, MMDS.; Martino, HSD.; Noratto, G.; Roque-Andrade, A.; Stringheta, PC.; Talcott, S.; Ramos, AM.; Mertens-Talcott, SU. Anti-inflammatory activity of polyphenolics difrom açai (Euterpe oleracea Martius) in intestinal myofibroblasts CCD-18Co cells. Food Funct 2015, 6(10): 3249–3256. https://doi.org/10.1039/c5fo00278h.
Rambey, R.; Tambunan, WA.; Hasibuan, M.; Siregar, FA.; Prayogo, B.; Silalahi, C.; Hasibuan, D.; Syahputra, N. Ethnobotany of the Arecaceae family in Torgamba District, South Labuhanbatu, North Sumatra. IOP Conf. Series: Earth Environ 2021, 1–5. https://doi.org/10.1088/1755-1315/782/3/032022.
Sundram, S. Growth effects by Arbuscular Mycorrhiza Fungi on oil palm (Elaeis guineensis Jacq.) seedlings. J Oil Palm Res 2010, 22: 796–802.
Wilson, C.; Tisdell, C. Why farmers continue to use pesticides despite environmental, health, and sustainability costs. Ecol Econ 2001, 39: 449–462. https://doi.org/10.1016/S0921-8009(01)00238-5.
Mateo-Sagasta, J.; Zadeh, SM.; Turral, H. Water pollution from agriculture – a global review. Food and Agriculture Organization of the United Nations Rome and the International Water Management Institute on behalf of the water land and ecosystems research program Colombo, 2017. https://www.fao.org/3/i7754e/i7754e.pdf.
John, DA.; Babu, GR. Lessons from the aftermaths of the Green Revolution on the food system and health. Front Sustain Food Syst 2021, 5: 644559. https://doi.org/10.3389/fsufs.2021.644559.
Neumeister, L. Foodwatch Report, Locked-in pesticides, the European Union's dependency on harmful pesticides, and how to overcome it, Brunnenstraße. Berlin, Germany; 2022
United Nations Environment Programme (UNEP), Synthesis report on the environmental and health impacts of pesticides and fertilizers and ways to minimize them, 2022; ISBN No: 978-92-807-3929-9. https://wedocs.unep.org/20.500.11822/38409.
Tamil Nadu Agricultural University (TNAU) Agrotech Portal, https://agritech.tnau.ac.in/agriculture/agri_nutrientmgt_coconut.html. Accessed on 26th September 2023.
Agrahari, P.; Kumar, N.; Pandey, N.; Sinku, S.; Khan, S.; Sahu, A.; Singh, VK.; Singh, DK. Phytoremediation of Lead contaminated soil with the help of Bambusa vulgaris. Alger J Biosciences 2023, 4(1): 064–070. https://doi.org/10.57056/ajb.v4i1.111
Yadegari, M.; Shamshiri, RR.; Shariff, ARM.; Balasundram, SK.; Mahns, B. Using spot-7 for nitrogen fertilizer management in oil palm. Agriculture 2020, 10: 133. https://doi.org/10.3390/agriculture10040133.
The Food and Agriculture Organization (FAO), Strategic Framework 2022-31. Food and Agriculture Organization of the United Nations, 2022. https://www.fao.org/3/cb7099en/cb7099en.pdf.
Hussaini, IM.; Ahmed, HS.; Ahmad, H.; Sulaiman, MA.; Usman, A. Preliminary screening for antibacterial activity of endophytic fungi isolated from Azadirachta indica and Mentha piperita against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Alger J Biosciences 2022, 3(2): 056–060. https://doi.org/10.57056/ajb.v3i2.57
Zaim, S.; Bekkar, AA. Advances in research on the use of Brevundimonas spp. to improve crop and soil fertility and for soil bioremediation. Alger J Biosciences 2023, 04(01): 045-051: https://doi.org/10.57056/ajb.v4i1.109
Brundrett, MC. Coevolution of roots and mycorrhizas of land plants. New Phytol 2002, 134: 275–304.
Janos, DP. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 2007, 17: 75–91. https://doi.org/10.1007/s00572-006-0094-1.
Motta, VD.; Munévar, MF. Response of oil palm seedlings to mycorrhization. Palmas 2005, 26 (3): 11–20. http://publicaciones.fedepalma.org/index.php/palmas/article/view/1136.
Rillig, MC.; Mummey, DL. Mycorrhizas and soil structure. New Phytol 2006, 171: 41–53. https://doi.org/10.1111/j.1469-8137.2006.01750.x.
Willis, A.; Rodrigues, BF.; Harris, PJC. The Ecology of Arbuscular Mycorrhizal Fungi. Crit Rev Plant Sci 2013, 32: 1–20. https://doi.org/10.1080/07352689.2012.683375.
Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223: 1115–1122. https://doi.org/10.1007/s00425-006-0225-0.
George, NP.; Ray, JG. The inevitability of arbuscular mycorrhiza for sustainability in organic agriculture — A critical review. Front Sustain Food Syst 2023, 10.3389: 1–23. https://doi.org/10.3389/fsufs.2023.1124688.
Khudairi, AK. Mycorrhiza in Desert Soils. BioScience 1969, 19(7): 598–599. https://doi.org/10.2307/1294933
Janos, DP. Vesicular-arbuscular mycorrhizae affect the growth of Bactris gasipaes. Principes 1977, 21: 12-18.
Lily, VG. Note on the development of vesicular-arbuscular mycorrhiza Endogone fasciculata in coconut root. Curr Sci 1975, 44: 201-202.
Blal, B.; Gianinazzi-Pearson, V. Interest in endomycorrhizae for the production of micro-propagated oil palm clones. Agric Ecosyst Environ 1989, 29: 39–43. https://doi.org/10.1016/0167-8809(90)90251-8.
Zougari-Elwedi, B.; Sanaa, M.; Labidi, S.; Sahraoui, etAL-H. Évaluation de l’ impact de la mycorhization arbusculaire sur la nutrition minérale des plantules de palmier dattier (Phœnix dactylifera L. var. Deglet Nour). Étude et Gestion Des Sols 2012, 19 (3&4): 193–202.
Almeida, DSde.; Freitas, MSM.; Carvalho, AJCde.; Beltrame, RA.; Moreira, SO.; Vieira, ME. Mycorrhizal fungi and phosphate fertilization in the production of Euterpe edulis seedlings. Rev Fac Cienc Agrar 2021, 53(2): 109–118. https://doi.org/10.48162/rev.39.045.
Rini, MV.; Suharjo, R.; Wibowo, L.; Irvanto, D.; Ariyanto, A. Seleksi empat jenis fungi mikoriza arbuskular pada bibit kelapa sawit yang ditanam pada tanah histosol. Menara Perkebunan 2021, 89 (1): 8–16. http://dx.doi.org/10.22302/iribb.jur.mp.v89i1.406.
Al-Karaki, GN. Application of mycorrhizae in sustainable date palm cultivation. Emir J Food Agric 2013, 25 (11): 854–862. https://doi.org/10.9755/ejfa.v25i11.16499.
Qaddoury, A. Arbuscular mycorrhizal fungi provide complementary characteristics that improve plant tolerance to drought and salinity: Date palm as a model. In Mycoremediation and Environmental Sustainability, Fungal Biology, Prasad, R., Eds.; Springer, 2017; pp. 189-215. https://doi.org/10.1007/978-3-319-68957-9_11.
Fisher, JB.; Jayachandran, K. Root structure and arbuscular mycorrhizal colonization of the palm Serenoa repens under field conditions. Plant Soil 1999, 217: 229–241. https://doi.org/10.1023/a:1004576001334.
Carrillo, LE.; Orellana, R.; Varela, L. Mycorrhizal Associations in Three Species of Palms of the Yucatan Peninsula, Mexico. Palms 2002, 46(1): 39–46.
Dreyer, B.; Morte, A.; López, JÁ.; Honrubia, M. Comparative study of mycorrhizal susceptibility and anatomy of four palm species. Mycorrhiza 2010, 20: 103–115. https://doi.org/10.1007/s00572-009-0266-x.
St.John, TV. Prospects for application of vesicular-arbuscular mycorrhizae in the culture of tropical palms. In Advances in Economic Botany, The Palm — Tree of Life: Biology, Utilization, and Conservation, New York Botanical Garden Press, 1988; pp. 50–55. https://www.jstor.org/stable/43927518.
Ali, ASR.; Dolmat, MT. Status of mycorrhizal research in oil palm, Oil Palm Bulletin; 1991. https://www.researchgate.net/publication/264749831_Status_of_Mycorrhizal_Research_in_Oil_Palm.
Naher, UA.; Othman, R.; Panhwar, QA. Beneficial effects of mycorrhizal association for crop production in the tropics-A review. Int J Agric Biol 2013, 15: 1021–1028. http://www.fspublishers.org/.
Akensous, FZ.; Anli, M.; Meddich, A. Biostimulants as innovative tools to boost date palm (Phoenix dactylifera L.) performance under drought, salinity, and heavy metal(oid) s' stresses: A concise review. Sustainability 2022, 14: 15984. https://doi.org/10.3390/su142315984.
Sengupta, A.; Chaudhuri, S. Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 2002, 12: 169–174. https://doi.org/10.1007/s00572-002-0164-y.
Bouamri, R.; Dalpé, Y.; Serrhini, MN.; Bennani, A. Arbuscular mycorrhizal fungi species associated with the rhizosphere of Phoenix dactylifera L. in Morocco. Afr J Biotechnol 2006, 5 (6): 510-516.
Ambili, K.; Thomas, GV.; Indu, P.; Gopal, M.; Gupta, A. Distribution of Arbuscular Mycorrhizae Associated with Coconut and Arecanut-Based Cropping Systems. Agric Res 2012, 1(4): 338–345. https://doi.org/10.1007/s40003-012-0036-4.
Rajeshkumar, PP.; Thomas, GV.; Gupta, A.; Gopal, M. Diversity, richness, and degree of colonization of arbuscular mycorrhizal fungi in coconut cultivated and intercrops in a highly productive zone of Kerala, India. Symbiosis 2015, 65: 125–141. https://doi.org/10.1007/s13199-015-0326-2.
Sundram, S.; Othman, R.; Idris, AS.; Angel, LPL.; Meon, S. Improved growth performance of Elaeis guineensis Jacq. through the applications of arbuscular mycorrhizal (AM) fungi and endophytic bacteria. Curr Microbiol 2022, 79: 155. https://doi.org/10.1007/s00284-022-02842-4.
Bennett, AE.; Grotten, K. The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annu Rev Plant Biol 2022, 73: 649-672. https://doi.org/10.1146/annurev-arplant-102820-124504.
Khan, AH.; Khan, KN.; Zubair, M.; Shaida, MA.; Manzar, MS.; Abutaleb, A.; Naushad, M.; Iqbal, J. Sustainable green nano adsorbents for remediation of pharmaceuticals from water and wastewater: A critical review. Environ Res 2022, 204: 112243. https://doi.org/10.1016/j.envres.2021.112243.
Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.; Smith, P.; Zeng, Z.; Olesen, JE.; Zang, H. Global systematic review with meta-analysis reveals the yield advantage of legume-based rotations and its drivers. Nat Commun 2022, 13: 4926. https://doi.org/10.1038/s41467-022- 32464-0.
Iyer, R.; Moosa, H.; Sastry, K. VA Mycorrhizal status of a coconut-based high-density multi-species cropping system, Coconut Research and Development, Central Plantation Crops Research Institute, Kerala, India; 1983, p 429-431.
Khaliel, AS.; Abou Heilah, AN. Formation of vesicular-arbuscular mycorrhizae in Phoenix dactylifera cultivated in Qasim region Saudi Arabia. Pak J Bot 1985, 17 (2): 267-270.
Thomas, GV.; Ghai, SK. Genotype dependent variation in vesicular-arbuscular mycorrhizal colonization of coconut seedlings. Proc Indian Acad Sci (Plant Sci) 1987, 97 (4): 289–294. https://doi.org/10.1007/BF03053382.
Thomas, GV.; Rajagopal, V.; Bopaiah, BM. VA-Mycorrhizal association in relation to drought tolerance in coconut. J Plant Crops 1993, 21: 98–103.
Auliana.; Kaonongbua, W. Preliminary study on biodiversity of arbuscular mycorrhizal fungi (AMF) in oil palm (Elaeis guineensis Jacq.) plantations in Thailand. IOP Conf. Ser.: Earth Environ Sci 2018, 144: 012010. https://dx.doi.org/10.1088/1755-1315/144/1/012010.
Rai, IN.; Suada, IK.; Proborini, MW.; Wiraatmaja, IW.; Semenov, M.; Krasnov, G. Indigenous endomycorrhizal fungi at salak (Salacca zalacca) plantations in Bali, Indonesia, and their colonization of the roots. Biodiversitas 2019, 20 (8): 2410–2416. https://doi.org/10.13057/biodiv/d200840.
Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mader, P.; Mliki, A.; Fki, L. Arbuscular mycorrhizal fungi associated with Phoenix dactylifera L. grown in Tunisian Sahara oases of different salinity levels. Symbiosis 2020, 81: 173–186. https://doi.org/10.1007/s13199-020-00692-x.
Gómez, SPM.; Berdugo, SEB.; Mena, RAM. Occurrence of indigenous arbuscular mycorrhizal fungi associated with the rhizosphere of the naidí palm in Colombia. Cienc Tecnol Agropecuaria 2020, 21 (3): e1275. https://doi.org/10.21930/rcta.vol21_num3_art:1275.
Ritaqwin, Z.; Maulana, M.; Nazalia. Identification of arbuscular mycorrhizae fungi on oil palm in Bireuen, Aceh. SEAS 2021, 5 (2): 114-121. http://dx.doi.org/10.22225/seas.5.2.3972.114-121.
Maia, RdaS.; Vasconcelos, SS.; Viana-Junior, AB.; Castellani, DC.; Kato, OR. Oil palm (Elaeis guineensis) shows higher mycorrhizal colonization when planted in agroforestry than in monoculture. Agrofor Syst 2021, 95: 731–740. https://doi.org/10.1007/s10457-021-00627-5.
Fabian, D.; Guadarrama, P.; Hernadez-Cuevas, L.; Ramos-Zapata, JA. Arbuscular mycorrhizal fungi in a coastal wetland in Yucatan, Mexico. Bot Sci 2018, 96 (1): 24–34. https://doi.org/10.17129/botsci.1216.
Ambili, K.; Thomas, GV.; Gopal, M.; Gupta, A. Influence of crop combinations and soil factors on diversity and association of arbuscular mycorrhizal fungi in arecanut-based cropping systems. J Plant Crops 2017, 45 (1): 20–32. https://www.researchgate.net/publication/317127620_Influence_of_crop_combinations_and_soil_factors_on_diversity_and_association_of_arbuscular_mycorrhizal_fungi_in_arecanut_based_cropping_systems.
Nobre, CP.; Gomes, M.; Goto, BT.; Gehring, C. Arbuscular mycorrhizal fungi associated with the babassu palm (Attalea speciosa) in the eastern periphery of Amazonia, Brazil. Acta Amazon 2018, 48 (4): 321–329. http://dx.doi.org/10.1590/1809-4392201800092.
Velazquez, MS.; Cabello, M.; Irrazabal, G.; Godeas, A. Acaulosporaceae from El Palmar National Park, Entre Ríos, Argentina. Mycotaxon 2008, 103: 171–187.
Velazquez, MS.; Biganzoli, F.; Cabello, MN. Arbuscular mycorrhizal fungi in El Palmar National Park (Entre Rios Province, Argentina) - A protected reserve. Sydowia 2010, 62: 149–163.
Velázquez, S.; Cabello, M. Occurrence and diversity of arbuscular mycorrhizal fungi in trap cultures from El Palmar National Park soils. Eur J Soil Biol 2011, 47: 230–235. https://doi.org/10.1016/j.ejsobi.2011.05.002.
Velázquez, MS.; Cabello, MN.; Barrera, M. Composition and structure of arbuscular-mycorrhizal communities in EL Palmar National Park, Argentina. Mycologia 2012, 105: 509–520. https://doi.org/10.3852/11-353.
Furrazola, E.; Sánchez-Rendón, JA.; Guadarrama, P.; Pernús, M.; Torres-Arias, Y. Mycorrhizal status of Coccothrinax crinita (Arecaceae), an endangered endemic species from western Cuba. Rev Mex Biodivers 2020, 91: e913048. https://doi.org/10.22201/ib.20078706e.2020.91.3048.
Lara-Pérez, LA.; Oros-Ortega, I.; Córdova-Iara, I.; Estrada-Medina, H.; O'Connor-Sánchez, A.; Góngora-Castillo, E.; Sáenz-Carbonell, L. Seasonal shifts of arbuscular mycorrhizal fungi in Cocos nucifera roots in Yucatan, Mexico. Mycorrhiza 2020, 30: 269-283. https://doi.org/10.1007/s00572-020-00944-0.
Gopal, M.; Arunachalam, AGV.; Maheswarappa, HP.; Thomas, GV.; Jacob, PM. Autochthonous nutrient recycling driven by soil microbiota could be sustaining high coconut productivity in Lakshadweep Islands sans external fertilizer application. World J Microbiol Biotechnol 2022, 38: 213. https://doi.org/10.1007/s11274-022-03373-7.
Nadarajah, P. Species of Endogonaceae and mycorrhizal association of Elaeis guineensis and Theobroma cacao. In Tropical mycorrhiza research, Mikola, P., Eds.; Clarendon Press, Oxford, 1980; pp. 232-237.
Nadarajah, P.; Nawawi, A. Mycorrhizal status of epiphytes in Malaysian oil palm plantations. Mycorrhiza 1993, 4: 21–25. https://doi.org/10.1007/BF00203246.
Rini, MV.; Yelli, F.; Tambunan, DL.; Damayanti, I. Morphological and molecular identifications of three native arbuscular mycorrhizal fungi isolated from the rhizosphere of Elaeis guineensis and Jatropha curcas in Indonesia. Biodiversitas 2021, 22 (11): 4940–4947. https://doi.org/10.13057/biodiv/d221128.
Asano, K.; Kagong, WVA.; Mohammad, SMB.; Sakazaki, K.; Talip, MSA.; Sahmat, SS.; Chan, MKY.; Isoi, T.; Kano-Nakata, M.; Ehara, H. Arbuscular mycorrhizal communities in the roots of sago palm in mineral and shallow peat soils. Agriculture 2021, 11: 1161. https://doi.org/10.3390/agriculture11111161.
Al-yahya'ei, MN.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of Southern Arabia. Mycorrhiza 2011, 21: 195–209. https://doi.org/10.1007/s00572-010-0323-5.
Symanczik, S.; Błaszkowski, J.; Chwat, G.; Boller, T.; Wiemken, A.; Al-Yahya'ei, MN. Three new species of arbuscular mycorrhizal fungi were discovered at one location in a desert of Oman: Diversispora omaniana, Septoglomus nakheelum, and Rhizophagus arabicus. Mycologia 2014, 106 (2): 243–259. https://doi.org/10.3852/106.2.243.
Symanczik, S.; Blaszkowski, J.; Koegel, S.; Boller, T.; Wiemken, A.; Al-yahya'Ei, MN. Isolation and identification of desert-habituated arbuscular mycorrhizal fungi newly reported from the Arabian Peninsula. J Arid Land 2014, 6 (4): 488–497. https://doi.org/10.1007/s40333-014-0021-9.
Bouamri, R.; Dalpé, Y.; Serrhini, MN. Effect of seasonal variation on arbuscular mycorrhizal fungi associated with date palm. Emir J Food Agric 2014, 26 (11): 977–986. https://doi.org/10.9755/ejfa.v26i11.18985.
Zougari-Elwedi, B.; Issami, W.; Msetra, A.; Sanaa, M.; Yolande, D.; Sahraoui, AL-H. Monitoring the evolution of the arbuscular mycorrhizal fungi associated with date palm. J New Sci 2016, 31 (12): 1822–1831. http://www.jnsciences.org/.
Meddich, A.; Mokhtar, MAEl.; Wahbi, S.; Boumezzough, etA. Évaluation des potentialités mycorhizogènes en lien avec les paramètres physico-chimiques des sols de palmeraies du Maroc (Marrakech et Ta fi lalet). Cah Agric 2017, 26: 45012. https://doi.org/10.1051/cagri/2017044.
Khirani, S.; Boutaj, H.; Modafar, Cel.; Khelil, AOE. Arbuscular mycorrhizal fungi associated with date palm in Ouargla region (Southeastern Algeria). Plant Cell Biotechnol Mol Biol 2020, 21 (45&46): 15–28.
Al-Yahya'ei, MN.; Błaszkowski, J.; Al-Hashmi, H.; Al-Farsi, K.; Al-Rashdi, I.; Patzelt, A.; Boller, T.; Wiemken, A.; Symanczik, S. From isolation to application: a case study of arbuscular mycorrhizal fungi of the Arabian Peninsula. Symbiosis 2021, 86: 123–132. https://doi.org/10.1007/s13199-021-00824-x.
Hilali, Rel.; Symanczik, S.; Kinany, Sel.; Oehl, F.; Ouahmane, L.; Bouamri, R. Cultivation, identification, and application of arbuscular mycorrhizal fungi associated with date palm plants in Drâa-Tafilalet oasis. Rhizosphere 2022, 22: 100521. https://doi.org/10.1016/j.rhisph.2022.100521.
Fisher, JB.; Jayachandran, K. Presence of arbuscular mycorrhizal fungi in South Florida native plants. Mycorrhiza 2005, 15: 580–588. https://doi.org/10.1007/s00572-005-0367-0.
Núñez-Castillo. O.; Álvarez-Sánchez, FJ. Arbuscular mycorrhizae of the palm Astrocaryum mexicanum in disturbed and undisturbed stands of a Mexican tropical forest. Mycorrhiza 2003, 13: 271–276. https://doi.org/10.1007/s00572-003-0231-z.
Junior, JPdaS.; Cardoso, EJBN. Micorriza arbuscular em cupuaçu e pupunha cultivados em sistema agroflorestal e em monocultivo na Amazônia Central. Pesqui Agropecu Bras 2006, 41 (5): 819–825. http://dx.doi.org/10.1590/S0100-204X2006000500014.
Dreyer, B.; Morte, A.; Pe´rez-Gilabert, M.; Honrubia, M. Autofluorescence detection of arbuscular mycorrhizal fungal structures in palm roots : an underestimated experimental method., Mycol Res 2006, 110: 887–897. https://doi.org/10.1016/j.mycres.2006.05.011
Zhao, ZW.; Xia, YM.; Qin, XZ.; Li, XW.; Cheng, LZ.; Sha, T.; Wang, GH. Arbuscular mycorrhizal status of plants and the spore density of arbuscular mycorrhizal fungi in the tropical rain forest of Xishuangbanna, southwest China. Mycorrhiza 2001, 11: 159–162. https://doi.org/10.1007/s005720100117.
Ramos-Zapata, JA.; Orellana, R.; Allen, EB. Mycorrhizal dynamics and dependence of Desmoncus orthacanthos Martius (Arecaceae), a native palm of the Yucatan Peninsula, Mexico. Interciencia 2006, 31: 364-370.
Galindo-Castaneda, T.; Romero, HM. Mycorrhization in oil palm (Elaeis guineensis and E. oleifera x E. guineensis) in the pre-nursery stage. Agron Colomb 2013, 31 (1): 95–102.
Rini, MV.; Pertiwi, KO.; Saputra, H. Selection of five arbuscular mycorrhizal fungi isolates for palm oil (Elaeis guineensis Jacq.) in nurseries. J Trop Agrotech 2017, 5 (3): 138-143.
Alizadeh, F.; Abdullah, SNA.; Khodavandi, A. Influence of Oil palm-fungi interactions on soil microfungal community and growth profile of the plant. J Pure Appl Microbiol 2013, 7 (4): 2577–2590.
Kartika, E.; Duaja, MD.; Gusniwati. Oil Palm (Elaeis guineensis) responses to indigenous mycorrhizae and cow manure in ultisol. Planta Tropika 2019, 7 (2): 103–109. https://doi.org/10.18196/pt.2019.099.103-109.
Rini, MV.; Yansyah, MP.; Arif, MAS. The application of arbuscular mycorrhizal fungi reduced the required dose of compound fertilizer for oil palm (Elaeis Guineensis Jacq.) in a nursery. IOP Conf. Series: Earth Environ 2022, 1012: 012011. https://doi.org/10.1088/1755-1315/1012/1/012011.
Zangaro, W.; Nisizaki, SMA.; Domingos, JCB.; Nakano, EM.; Zangaro, W.; Nisizaki, SMA.; Domingos, JCB.; Nakano, EM. Mycorrhizal response and successional status in 80 woody species from south Brazil. J Trop Ecol 2003, 19: 315–324. https://doi.org/10.1017/S0266467403003341.
Hilali, Rel.; Bouamri, R.; Crozilhac, P.; Calonne, M.; Symanczik, S.; Ouahmane, L.; Hilali, Rel. In vitro colonization of date palm plants by Rhizophagus irregularis during the rooting stage. Symbiosis 2021, 84: 83–89.
Lugo, MA.; Giordano, PG.; Urcelay, C.; Crespo, EM. Root colonization by fungal endophytes in Trithrinax campestris (Arecaceae) from semiarid ecosystems from Central Argentine. Bol Soc Argent Bot 2011, 46 (3-4): 213-222.
Rajkumar, HG.; Seema, HS.; Sunil, KCP. Diversity of arbuscular mycorrhizal fungi associated with some medicinal plants in Western Ghats of Karnataka region, India. J Sci Technol 2012, 2(1): 13–20.
Belay, Z.; Vestberg, M.; Assefa, F. Diversity and abundance of arbuscular mycorrhizal fungi associated with acacia trees from different land use systems in Ethiopia. Afr J Microbiol Res 2013, 7(48): 5503–5515. https://doi.org/10.5897/ajmr2013.6115.
Chang-cong, L.; Su-ye, Z.; Lei, L.; Jun-sheng, H. Arbuscular mycorrhizal fungi associated with common tree species in a tropical rain forest in Bawangling of Hainan Island in China. Chin J Ecol 2010, 2: 269–273.
Li, X.; Gai, J.; Cai, X.; Li, X. Molecular diversity of arbuscular mycorrhizal fungi associated with two co-occurring perennial plant species on a Tibetan altitudinal gradient. Mycorrhiza 2013, 1–13. https://doi.org/10.1007/s00572-013-0518-7.
Wang, M.; Jiang, P. Colonization and diversity of AM fungi by morphological analysis on medicinal plants in Southeast China. Sci World J 2015, 753842: 1-8.
Liu, H.; Wang, Y.; Tang, M. Arbuscular mycorrhizal fungi diversity associated with two halophytes, Lycium barbarum L. and Elaeagnus angustifolia L. in Ningxia, China. Arch Agron Soil Sci 2017, 63(6): 796–806. https://doi.org/10.1080/03650340.2016.1235783
Wang, J.; Wang, GG.; Zhang, B.; Yuan, Z.; Fu, Z.; Yuan, Y.; Zhu, L.; Ma, S.; Zhang, J. Arbuscular mycorrhizal fungi associated with tree species in a planted forest of Eastern China. Forests 2019, 10(424): 1–14.
Wang, B.; Qiu, YL. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16(5): 299–363. https://doi.org/10.1007/s00572-005-0033-6.
Mayakrishnan, B.; Ravichandran, KR.; Thangavelu, M. Fine root endophyte association in widely cultivated palms of southern India. Kavaka 2022, 58(3): 48–53. https://doi.org/10.36460/Kavaka/58/3/2022/48-53.
Heijden, MGA.; Martin, FM.; Selosse, MA.; Sanders, IR. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol 2015, 205: 1406–1423. https://doi.org/10.1111/nph.13288.
Liu, L.; Yan, W.; Liu, B. Transcriptome sequencing of Cocos nucifera leaves in response to Rhynchophorus ferrugineus infestation. Front Genet 2023, 1–13. https://doi.org/10.3389/fgene.2023.1115392
Janos, DP. Mycorrhiza applications in tropical forestry: are temperate-zone approaches appropriate? In Trees and mycorrhiza, Ng, FSP., Eds.; Forest Research Institute, Kuala Lumpur, Malaysia,1988; pp. 133–188.
Siqueira, JO.; Saggin-Júnior, OJ. Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 2001, 11(5): 245–255. https://doi.org/10.1007/s005720100129.
Lies, A.; Prin, Y.; Duponnois, R.; Ferhout, H. The Management of the Mycorrhizal Soil Infectivity: Ecological and Technical Approaches. In Mycorrhiza - Eco-Physiology, Secondary Metabolites, Nanomaterials, Varma, A., Prasad, R., Tuteja, N., Eds.; Springer, 2017; pp. 209–221. https://doi.org/10.1007/978-3-319-57849-1.
Bubici, G.; Kaushal, M.; Prigigallo, MI.; Cabanás, CGL.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front Microbiol 2019, 10: 616. https://doi.org/10.3389/fmicb.2019.00616.
Yang, Y.; Liang, Y.; Han, X.; Chiu, TY.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci Rep 2016, 6: 20469. https://doi.org/10.1038/srep20469.
Wu, S.; Shi, Z.; Huang, M.; Li, Y.; Gao, J. Effects of Arbuscular Mycorrhizal Fungi on Leaf N:P: K Stoichiometry in Agroecosystem. Agronomy 2023, 13: 358 https://doi.org/10.3390/agronomy13020358.
Chandrasekaran, M. A meta-analytical approach on arbuscular mycorrhizal fungi inoculation efficiency on plant growth and nutrient uptake. Agriculture 2020, 10: 370. https://doi.org/10.3390/agriculture10090370.
Ilangamudali, IMPS.; Senarathne, SHS. Effectiveness of arbuscular mycorrhizal fungi-based biofertilizer on early growth of coconut seedlings. Cocos 2016, 22: 1-12. https://doi.org/10.4038/cocos.v22i1.5807.
Sulistiono, W.; Brahmantiyo, B.; Hartanto, S.; Aji, HB.; Bina, HK. Effect of arbuscular mycorrhizal fungi and NPK fertilizer on roots growth and nitrate reductase activity of coconut. J Agron 2020, 19 (1): 46–53. https://doi.org/10.3923/ja.2020.46.53.
Diatta, ILD.; Kane, A.; Agbangba, CE.; Sagna, M.; Diouf, D.; Aberlenc-Bertossi, F.; Duval, Y.; Borgel, A.; Sane, D. Inoculation with arbuscular mycorrhizal fungi improves seedling growth of two Sahelian date palm cultivars (Phoenix dactylifera L., cv. Nakhla hamra and cv. Tijib) under salinity stresses. Adv Biosci Biotechnol 2014, 5: 64–72. http://dx.doi.org/10.4236/abb.2014.51010.
Benhiba, L.; Fouad, MO.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palms subjected to long-term drought. Trees 2015, 29: 1725-1733. https://doi.org/10.1007/s00468-015-1253-9.
Boutheina, Z-E.; Aya, H.; Naima, B.; Ahmed, N. Responses of date Palm seedling to co-inoculation with phosphate solubilizing bacteria and mycorrhizal arbuscular fungi. Int J Environ Agric Biotech 2019, 4 (2): 581–588. http://dx.doi.org/10.22161/ijeab/4.2.43.
Ait-El-Mokhtar, M.; Laouane, RB.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci Hortic 2019, 253: 429–438. https://doi.org/10.1016/j.scienta.2019.04.066.
Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Rahou, YA.; Chitt, MA.; Oufdou, K.; Mitsui, T.; Hafidi, M.; Meddich, A. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci 2020, 11: 516818. https://doi.org/10.3389/fpls.2020.516818.
Anli, M.; Kaoua, MEL.; Ait-El-Mokhtar, M.; Boutasknit, A.; Ben-Laouane, R.; Toubali, S.; Baslam, M.; Lyamlouli, K.; Hafidi, M.; Meddich, A. Seaweed extract application and arbuscular mycorrhizal fungal inoculation: a tool for promoting growth and development of date palm (Phoenix dactylifera L.) cv «Boufgous». S Afr J Bot 2020, 132: 15–21. https://doi.org/10.1016/j.sajb.2020.04.004.
Anli, M.; Symanczik, S.; Abbassi, Ael.; Ait-El-Mokhtar, M.; Boutasknit, A.; Ben-Laouane, R.; Toubali, S.; Baslam, M.; Mader, P.; Hafidi, M.; Meddich, A. Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera "Boufgouss." Plant Biosyst 2020, 155: 763-771. https://doi.org/10.1080/11263504.2020.1779848.
Raho, O.; Boutasknit, A.; Anli, M.; Ben-Laouane, R.; Rahou, YA.; Ouhaddou, R.; Duponnois, R.; Douira, A.; Modafar, CE.; Meddich, A. Impact of native biostimulants/ biofertilizers and their synergistic interactions on the agro-physiological and biochemical responses of date palm seedlings. Gesunde Pflanz 2022, 74: 1053-1069. https://doi.org/10.1007/s10343-022-00668-5.
Rini, MV.; Efriyani, U. Respons bibit kelapa sawit (Elaeis guineensis Jacq.) terhadap pemberian fungi mikoriza arbuskular dan cekaman air. Menara Perkebunan 2016, 84 (2): 106–114. https://doi.org/10.22302/iribb.jur.mp.v84i2.225.
Gallaud, I. Études sur les mycorrhizes Endotrophs. le Bigot Frères, Lille, France: 1904.
Dickson, S. The Arum – Paris continuum of mycorrhizal symbioses. New Phytol 2004, 163: 187–200. https://doi.org/10.1111/j.1469-8137.2004.01095.x.
Smith, FA.; Smith, SE. Structural diversity in (vesicular)-arbuscular mycorrhizal symbiosis. New Phytol 1997, 137: 373-388
Rahim, NA.; Jais, HM.; Hassan, HM. Environment and host affects Arbuscular Mycorrhiza Fungi (AMF) population. Trop Life Sci Res 2016, 27(2010): 9–13. https://doi.org/10.21315/tlsr2016.27.3.2.
Dreyer, B.; Honrubia, M.; Morte, A. How root structure defines the Arbuscular Mycorrhizal Symbiosis and what we can learn from it? In Root Engineering, Soil Biology, Morte, A., Varma, A., Eds.; Springer-Verlag Berlin Heidelberg, 2014; pp. 145–169. https://doi.org/10.1007/978-3-642-54276-3.
Gutjahr, C.; Casieri, L.; Paszkowski, U. Glomus intraradices induce changes in the root system architecture of rice independently of common symbiosis signaling. New Phytol 2009, 182: 829–37. https://doi.org/10.1111/j.1469-8137.2009.02839.x.
Gutjahr, C.; Siegler, H.; Haga, K.; Lino, M.; Paszkowski, U. Full establishment of arbuscular mycorrhizal symbiosis in rice occurs independently of enzymatic jasmonate biosynthesis. PLoS One 2015, 10: e0123422. https://doi.org/10.1371/journal.pone.0123422.
Bernaola, L.; Cosme, M.; Schneider, RW.; Stout, M. Belowground inoculation with Arbuscular mycorrhizal fungi increases the local and systemic susceptibility of rice plants to different pest organisms. Front Plant Sci 2018, 9: 747. https://doi.org/10.3389/fpls.2018.00747.
Fiorilli, V.; Vallino, M.; Biselli, C.; Faccio, A.; Bangaresi, P.; Bonfante, P. Host and non-host roots in rice: cellular and molecular approaches reveal differential responses to arbuscular mycorrhizal fungi. Front Plant Sci 2015, 6: 636. https://doi.org/10.3389/fpls.2015.00636.
Clement, CR.; Habte, M. Genotypic variation in vesicular‐arbuscular mycorrhizal dependence of the pejibaye palm. J Plant Nutr 1995, 18 (9): 1907-1916. https://doi.org/10.1080/01904169509365032.
Shukla, A.; Kumar, A.; Jha, A.; Dhyani, SK.; Vyas, D. Cumulative effects of tree-based intercropping on arbuscular mycorrhizal fungi. Biol Fertil Soils 2012, 48(8): 899–909. https://doi.org/10.1007/s00374-012-0682-5.
Bini, D.; Santos, Cados.; Silva, MCPda.; Bonfim, JA.; Cardoso, EJBN.; Andreote, FD. Intercropping Acacia mangium stimulates AMF colonization and soil. Sci Agric 2018, 75(2): 102–110. http://dx.doi.org/10.1590/1678-992X-2016-0337.
Ou-Zine, M.; Symanczik, S.; Kinany, S EI.; Aziz, L.; Fagroud, M.; Abidar, A.; Mäder, P.; Achbani, EI H.; Haggoud, A.; Hilali, R EL.; Abdellaoui, M.; Bouamri, R. Effect of PGPR and mixed cropping on mycorrhizal status, soil fertility, and date palm productivity under organic farming system. Res Sq 2023, 1–16. https://doi.org/10.21203/rs.3.rs-3225865/v1.
Abbott, LK.; Robson, AD. Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric Ecosyst Environ 1991, 35: 121–150. https://doi.org/10.1016/0167-8809(91)90048-3.
Bohrer, KE.; Friese, CF.; Amon, JP. Seasonal dynamics of arbuscular mycorrhizal fungi in differing wetland habitats. Mycorrhiza 2004, 14: 329–337. https://doi.org/10.1007/s00572-004-0292-7.
Sankaralingam, A.; Hemalatha, G.; Ali, AM. A Treatise on Palmyrah. Central plantation crops research institute, Kasaragod, Kerala; 1999.
Renuka, C.; Bhat, KV.; Basha, SC. Palm Resources of Kerala and Their Utilisation, Kerala Forest Research Institute Peechi, Thrissur; 1996, 116: 31 https://www.cabdirect.org/cabdirect/abstract/19980614676.
Sheshrao, DU.; Gyananath, G. Seasonal Variation of spore density and root colonisation of Arbuscular Mycorrhizae in crop plants in relation to soil edaphic factors. In Prospects in Bioscience: Addressing the Issues, Sabu, A., Augustine, A., Eds.; Springer, India, 2013; pp. 141–149. https://doi.org/10.1007/978-81-322-0810-5.
Bhardwaj, AK.; Chandra, KK. Soil moisture fluctuation influences AMF root colonization and spore population in tree species planted in degraded entisol soil. Int J Biosci 2018, 13(3): 229–243. https://doi.org/10.12692/ijb/13.3.229-243
Escudero, V.; Mendoza, R. Seasonal variation of arbuscular mycorrhizal fungi in temperate grasslands along a wide hydrologic gradient. Mycorrhizae 2005, 15: 291–299. https://doi.org/10.1007/s00572-004-0332-3.
Hodel, DR.; Pittenger, DR.; Downer, AJ. Palm root growth and implications for transplanting. Arboric J 2005, 31(4): 171–181. https://doi.org/10.48044/jauf.2005.022.
Torti, SD.; Coley, PD.; Janos, DP. Vesicular-Arbuscular Mycorrhizae in Two Tropical Monodominant Trees. J Trop Ecol 1997, 13(4): 623–629.
Zubek, S.; Kapusta, P.; Rożek, K.; Błaszkowski, J.; Gielas, I.; Nobis, M.; Świerszcz, S.; Nowak, A. Fungal root colonization and arbuscular mycorrhizal fungi diversity in soils of grasslands with different mowing intensities. Appl Soil Ecol 2022, 172. https://doi.org/10.1016/j.apsoil.2021.104358.
Cássia-Silva, C.; Oliveira, RS.; Sales, LP.; Freitas, CG.; Jardim, L.; Emilio, T.; Bacon, CD.; Collevatti, RG. Acaulescence promotes speciation and shapes the distribution patterns of palms in Neotropical seasonally dry habitats. Ecography 2022, (3): 1–13. https://doi.org/10.1111/ecog.06072.
Morton, JB.; Albers, V. The arbuscular mycorrhizal symbiosis and its role in date palm production and sustainability. Proceedings of the Fifth International Date Palm Conference, 2009; pp. 371–380.
Baylis, GTS. The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In Endomycorrhizas, Sanders, FE., Mosse, B., Tinker, PB., Eds.; Academic Press, London, UK, 1975; pp. 373– 389.
Akensous, FZ.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Ait-Rahou, Y.; Ahmed, HB.; Nasri, N.; Hafidi, M.; Meddich, A. Boosting date palm (Phoenix dactylifera L.) growth under drought stress: effects of innovative biostimulants. Gesunde Pflanz 2022, 74: 961–982. https://doi.org/10.1007/s10343-022-00651-0.
Gómez‑Falcón, N.; Carbonell, LAS.; Torres, AA.; Pérez, LAL.; Oropeza, MNC. Arbuscular mycorrhizal fungi increase the survival and growth of micro-propagated coconut (Cocos nucifera L.) plantlets. In Vitro Cell Dev Biol Plant 2023, 59 (3): 401- 412. https://doi.org/10.1007/s11627-023-10345-5.
Fisher, JB.; Jayachandran, K. Beneficial role of arbuscular mycorrhizal fungi on Florida native palms. Palms 2008, 52 (3): 113-123.
Sgrott, AF.; Booz, MR.; Pescador, R.; Heck, TC; Stürmer, SL. Arbuscular mycorrhizal inoculation increases the biomass of Euterpe edulis and Archontophoenix alexandrae after two years under field conditions. Rev Bras Ciênc Solo 2012, 36: 1103–1112.
Ramos-Zapata, JA.; Orellana, R.; Allen, EB. Establishment of Desmoncus orthacanthos Martius (Arecaceae): Effect of inoculation with arbuscular mycorrhizae. Rev Biol Trop 2006, 54 (1): 65–72.
Ramos-Zapata, J.; Orellana, R.; Guadarrama, P.; Medina-Peralta, S. Contribution of mycorrhizae to early growth and phosphorus uptake by a neotropical palm. J Plant Nutr 2009, 32: 855–866. https://doi.org/10.1080/01904160902790333.
Blal, B.; Morel, C.; Gianinazzi-Pearson, V.; Fardeau, JC.; Gianinazzi, S. Influence of vesicular-arbuscular mycorrhizae on phosphate fertilizer efficiency in two tropical acid soils planted with micro propagated oil palm (Elaeis guineensis Jacq.). Biol Fertil Soils 1990, 9: 43–48. https://doi.org/10.1007/BF00335860.
Schultz, C. Effect of (vesicular) arbuscular mycorrhiza on survival and post vitro development of micro propagated oil palms (Elaeis guineensis Jacq.). Dissertation, Georg-August University, Goettingen, 2001.
Khodavandi, A.; Alizadeh, F. Gene expression profiling of fatty acid biosynthetic pathway during interaction of oil palm (Elaeis guineensis Jacq.) with the mutualistic fungus Glomus etunicatum. Acta Physiol Plant 2015, 37: 221. https://doi.org/10.1007/s11738-015-1970-0.
Krisnarini, Rini, MV.; Timotiwu, PB. The growth of oil palm (Elaeis guineensis Jacq.) seedlings with the application of different arbuscular mycorrhiza fungi and various phosphorous dosages. J Trop Soils 2018, 23 (3): 117–124. https://doi.org/10.5400/jts.2018.v23i3.117.
Duaja, MD.; Kartika, E.; Lizawati. Application of indigenous AMF from ex-coal mining soil combined with phosphorus fertilizers to improved oil palm seedling growth (Elaeis guineensis Jacq.). Biogenesis 2019, 7 (1): 38–43. https://doi.org/10.24252/bio.v7i1.5990.
Chu, EY. The effects of arbuscular mycorrhizal fungi inoculation on Euterpe oleracea Mart. Seedlings. Pesqui Agropecu Bras 1999, 34 (6): 1019–1024. https://doi.org/10.1590/s0100-204x1999000600013.
Al-Whaibi, MH.; Khaliel, AS. The effect of Mg on Ca, K, and P content of date palm seedlings under mycorrhizal and non-mycorrhizal conditions. Mycoscience 1994, 35: 213–217. https://doi.org/10.1007/BF02268440.
Jaiti, F.; Meddich, A.; Hadrami, I EL. Effectiveness of arbuscular mycorrhizal fungi in the protection of date palm (Phoenix dactylifera L.) against bayoud disease. Physiol Mol Plant Pathol 2007, 71: 166–173. https://doi.org/10.1016/j.pmpp.2008.01.002.
Baslam, M.; Qaddoury, A.; Goicoechea, N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological, and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 2013, 28: 161-172. https://doi.org/10.1007/s00468-013-0939-0.
Meddich, A.; Jaiti, F.; Bourzik, W.; Asli, A El.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palms (Phoenix dactylifera). Sci Hortic 2015, 192: 468–474. https://doi.org/10.1016/j.scienta.2015.06.024.
Meddich, A.; Oihabi, A.; Jaiti, F.; Bourzik, W.; Hafidi, M. Rôle des champignons mycorhiziens arbusculaires dans la tolérance du palmier dattier (Phoenix dactylifera) a` la fusariose vasculaire et au déficit hydrique. Botany 2015, 93: 1–9. http://dx.doi.org/10.1139/cjb-2014-0249.
Meddich, A.; Mokhtar, MAEl.; Bourzik, W.; Mitsui, T.; Baslam, M.; Hafid, M. Optimizing growth and tolerance of date palm (Phoenix dactylifera L.) to drought, salinity, and vascular fusarium-induced wilt (Fusarium oxysporum) by application of arbuscular mycorrhizal fungi (AMF). In Root Biology, Giri, B., Prasad, R., Varma, A., Eds.; Springer, 2018; pp. 239–258. https://doi.org/10.1007/978-3-319-75910-4.
Kinany, Sel.; Achbani, E.; Faggroud, M.; Ouahmane, L.; Hilali, Rel.; Haggoud, A.; Bouamri, R. Effect of organic fertilizer and commercial arbuscular mycorrhizal fungi on the growth of micro propagated date palm cv. Feggouss. J Saudi Soci Agric Sci 2018, 18: 411–417. https://doi.org/10.1016/j.jssas.2018.01.004.
Faghire, M.; Samri, S.; Baslam, M.; Goicoechea, N.; Meddich, A.; Qaddoury, A. Positive effects of arbuscular mycorrhizal fungi on biomass production, nutrient status, and water relations in date palm seedlings under water deficiency. Acta Hort 2010, 882: 833–838.
Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and /or compost. Front Sustain Food Syst 2020, 4: 131. https://doi.org/10.3389/fsufs.2020.00131.
Kazadi, AT.; wa Lwalaba, JL.; Ansey, BK.; Muzulukwau, JM.; Katabe, GM.; Karul, MI.; Baert, G.; Haesaert, G.; Mundende, RPM. Effect of phosphorus and Arbuscular Mycorrhizal Fungi (AMF) inoculation on growth and productivity of Maize (Zea mays L.) in a Tropical Ferralsol. Gesunde Pflanzen 2022, 74: 159–165 (2022). https://doi.org/10.1007/s10343-021-00598-8
Ma, X.; Geng, Q.; Zhang, H.; Bian, C.; Chen, HYH.; Jiang, D.; Xu, X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity, and ecosystem multifunctionality. New Phytol 2021, 229(5): 2957–2969. https://doi.org/10.1111/nph.17077.
Vance, CP.; Chiou, TJ. Phosphorus focus editorial. Plant Physiol 2011, 156(3): 987–988. https://doi.org/10.1104/pp.111.900415.
Elser, JJ. Phosphorus: a limiting nutrient for humanity? Curr Opin Biotechnol 2012, 23(6): 833–838.
Wang, S.; Song, M.; Wang, C.; Dou, X.; Wang, X.; Li, X. Mechanisms underlying soil microbial regulation of available phosphorus in a temperate forest exposed to long-term nitrogen addition. Sci Total Environ 2023, 18 (904): 166403. doi: https://doi.org/10.1016/j.scitotenv.2023.166403.
Phosri, C.; Rodriguez, A.; Sanders, IR.; Jeffries, P. The role of mycorrhizas in more sustainable oil palm cultivation. Agric Ecosyst Environ 2010, 135: 187–193. https://doi.org/10.1016/j.agee.2009.09.006.
Janos, DP. Vesicular-Arbuscular Mycorrhizae Affect Lowland Tropical Rain Forest Plant Growth. Ecology 1980, 61(1): 151–162.
Peat, AHJ.; Fitter, AH. The distribution of arbuscular mycorrhizas in the British flora. New Phytol 1993, 125(4): 845–854.
Jin, L.; Wang, S.; Wang, X.; Shen, Y. Seed size influences arbuscular mycorrhizal symbiosis across leguminous host-plant species at the seedling stage. Symbiosis 2009, 49(2): 111–116. https://doi.org/10.1007/s13199-009-0013-2.
Janos, DP.; Schroeder, MS.; Schaffer, B.; Crane, JH. Inoculation with arbuscular mycorrhizal fungi enhances the growth of Litchi chinensis Sonn. trees after propagation by air-layering. Plant Soil 2001, 233(1): 85–94. https://doi.org/10.1023/A:1010329618152.
Lehmann, A.; Rillig, MC. Arbuscular mycorrhizal contribution to crops' copper, manganese and iron nutrient concentrations-A meta-analysis. Soil Biol Biochem 2015, 81: 147–158. https://doi.org/10.1016/j.soilbio.2014.11.013.
Ahanger, MA.; Hashem, A.; Abd-Allah, EF.; Ahmad, P. Arbuscular Mycorrhiza in crop improvement under environmental stress. In Emerging Technologies and Management of Crop Stress Tolerance, Ahmad, P., Eds.; Elsevier, 2014; PP. 69-95 https://doi.org/10.1016/B978-0-12-800875-1.00003-X.
Hazzouri, KM.; Flowers, JM.; Nelson, D.; Lemansour, A.; Masmoudi, K.; Amiri, KMA. Prospects for the study and improvement of abiotic stress tolerance in date palms in the post-genomics Era. Front Plant Sci 2020, 11: 293. https://doi.org/10.3389/fpls.2020.00293.
Sivakumar, V.; Sudha, R.; Niral, V.; Praneetha, S. Drought: Effects, mechanisms, and mitigation strategies in coconut. Indian Coconut Journal 2021, 5: 17–20.
Outamamat, E.; Bourhia, M.; Dounas, H.; Salamatullah, AM.; Alzahrani, A.; Alyahya, HK.; Albadr, NA.; Najib, M.; Feddy, A.; Mnasri, B.; Ouahmane, L. Application of native or exotic arbuscular mycorrhizal fungi complexes and monospecific isolates from saline semiarid Mediterranean ecosystems improved Phoenix dactylifera's growth and mitigated salt stress negative effects. Plants 2021, 10: 2501. https://doi.org/10.3390/plants10112501.
Yaish, MW.; Kumar, PP. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front Plant Sci 2015, 6: 348. https://doi.org/10.3389/fpls.2015.00348.
Hashem, A.; Abd_Allah, EF.; Alqarawi, AA.; Egamberdieva, D. Arbuscular Mycorrhizal Fungi and plant stress tolerance. In Plant Microbiome: Stress Response, Microorganisms for Sustainability, Egamberdieva, D., Ahmad, P., Eds.; Springer Nature, Singapore, 2018; pp. 81–103. https://doi.org/10.1007/978-981-10-5514-0_4.
Harkousse, O.; Slimani, A.; Jadrane, I.; Aitboulahsen, M.; Mazri, MA.; Zouahri, A.; Ouahmane, L.; Koussa, T.; Feddy, MNA. Role of local biofertilizer in enhancing the oxidative stress defense systems of date palm seedlings (Phoenix dactylifera L.) against abiotic stress. Appl Environ Soil Sci 2021, 6628544. https://doi.org/10.1155/2021/6628544.
Sembiring, M.; Jefri, Sakiah.; Wahyuni, M. The inoculation of mycorrhiza and Talaromyces pinophilus toward improving growth and phosphorus uptake of oil palm seedlings (Elaeis guineensis Jacq) on saline soil media. Bulg J Agric Sci 2018, 24 (4): 617–622.
Naser, HM.; Hanan, El-H.; Elsheery, NI.; Kalaji, HM. Effect of biofertilizers and putrescine amine on the physiological features and productivity of date palm (Phoenix dactylifera L.) grown on reclaimed-salinized soil. Trees 2016, 30: 1149–1161. https://doi.org/10.1007/s00468-016-1353-1.
Toubali, S.; Tahiri, A.; Anli, M.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Oufdou, K.; Ait-Rahou, Y.; Ben-Ahmed, H.; Jemo, M.; Hafidi, M. Meddich, A. Physiological and biochemical behaviors of date palm vitroplants treated with microbial consortia and compost in response to salt stress. Appl Sci 2020, 10: 8665. http://dx.doi.org/10.3390/app10238665.
Rajendran, R.; Esakkimuthu, R.; Kandasamy, V.; Babu, M.; Maheswarappa, HP. Identification and confirmation of hotspot areas and management of root (wilt) disease in coconut. Phytopathogenic Mollicutes 2019, 9 (2): 270–277. https://doi.org/10.5958/2249-4677.2019.00125.7.
Sujarit, K.; Pathom-aree, W.; Mori, M.; Dobashi, K.; Shiomi, K.; Lumyong, S. Streptomyces palmae CMU-AB204T, an antifungal producing-actinomycete, is a potential biocontrol agent to protect palm oil-producing trees from basal stem rot disease fungus, Ganoderma boninense. Biol Control 2020, 148: 104307. https://doi.org/10.1016/j.biocontrol.2020.104307.
Egonyu, JP.; Baguma, J.; Mart, LC.; Priwiratama, H.; Subramanian, S.; Tanga, CM.; Anankware, JP.; Roos, N.; Niassy, S. Global advances on insect pest management research in Oil Palm. Sustainability 2022, 14(16288): 1–24.
Berdeni, D.; Cotton, TEA.; Daniell, TJ.; Bidartondo, MI. The effects of Arbuscular Mycorrhizal Fungal colonisation on nutrient status, growth, productivity, and canker resistance of Apple (Malus pumila). Front in Micro 2018, 9(1461): 1–15. https://doi.org/10.3389/fmicb.2018.01461.
Boutaj, H.; Chakhchar, A.; Meddich, A.; Wahbi, S.; ElZ.; Talibi, A. Bioprotection of olive tree from Verticillium wilt by autochthonous endomycorrhizal fungi. J Plant Dis Prot 2020, 127: 3. https://doi.org/10.1007/s41348-020-00323-z.
Zhu, B.; Gao, T.; Zhang, D.; Ding, K.; Li, C. Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci Hortic 2022, 303: 1–5. https://doi.org/10.1016/j.scienta.2022.111219.
Gough, EC.; Owen, KJ.; Zwart, RS.; Thompson, JP. A systematic review of the effects of Arbuscular Mycorrhizal Fungi on root-lesion nematodes, Pratylenchus spp. Front Plant Sci 2020, 11(923): 1–14. https://doi.org/10.3389/fpls.2020.00923.
Sundram, S.; Meon, S.; Seman, IA.; Othman, R. Symbiotic interaction of endophytic bacteria with arbuscular mycorrhizal fungi and its antagonistic effects on Ganoderma boninense. J Microbiol 2011, 49 (4): 551–557. https://doi.org/10.1007/s12275-011-0489-3.
Sundram, S.; Meon, S.; Seman, IA.; Othman, R. Application of arbuscular mycorrhizal fungi with Pseudomonas aeruginosa UPMP3 reduces the development of Ganoderma basal stem rot disease in oil palm seedlings. Mycorrhiza 2015, 25: 387–397. https://doi.org/10.1007/s00572-014-0620-5.
Nurzannah, SE.; Purnamasari, I.; Siagian, DR.; Ramija, KEL. Potential of Trichoderma and Mycorrhizae as biological agents for controlling Ganoderma boninense in oil palm. IOP Conf. Series: Earth Environ Sci 2022, 974: 012097. https://doi.org/10.1088/1755-1315/974/1/012097.
Hendarjanti, H.; Sukorini, H. Controlling basal stem rot in oil palm plantations by applying arbuscular mycorrhizal fungi and Trichoderma spp. KnE Life Sci 2022, 7: 206–227. https://doi.org/10.18502/kls.v7i3.11121.
Jaiti, F.; Kassami, M.; Meddich, A.; Hadrami, I EL. Effect of arbuscular mycorrhization on the accumulation of hydroxycinnamic acid derivatives in date palm seedlings challenged with Fusarium oxysporum f. sp. albedinis. J Phytopathol 2008, 156: 641–646. https://doi.org/10.1111/j.1439-0434.2008.01411.x.
Abohatem, M.; Chakrafi, F.; Jaiti, F.; Dihazi, A.; Baaziz, M. Arbuscular mycorrhizal fungi limit the incidence of Fusarium oxysporum f.sp. albedinis on date palm seedlings by increasing nutrient contents, total phenols, and peroxidase activities. Horti J 2011, 4: 10–16. https://doi.org/10.2174/1874840601104010010.
Khaled, LB.; Pérez-Gilabert, M.; Dreyer, B.; Oihabi, A.; Honrubia, M.; Morte, A. Peroxidase changes in Phoenix dactylifera palms inoculated with mycorrhizal and biocontrol fungi. Agron Sustain Dev 2008, 28: 411-418. http://dx.doi.org/10.1051/agro:2008018.
Rini, MV.; Hasan, SN.; Hidayat, KF.; Aeny, TN. Applications of arbuscular mycorrhiza fungi to improve the growth of oil palm seedlings and disease resistance against Ganoderma sp. J Agric Sci Technol 2022, 6 (1): 31–40. https://doi.org/10.55043/jaast.v6i1.40.
Selvaraj, A.; Thangavel, K.; Uthandi, S. Arbuscular mycorrhizal fungi (Glomus intraradices) and diazotrophic bacterium (Rhizobium BMBS) primed defense in black gram against herbivorous insect (Spodoptera litura) infestation. Microbiol Res 2020, 231: 126355. https://doi.org/10.1016/j.micres.2019.126355.
Yu, L.; Zhang, W.; Geng, Y.; Liu, K.; Shao, X. Cooperation with Arbuscular Mycorrhizal Fungi increases plant nutrient uptake and improves defenses against insects. Front Ecol Evol 2022, 10: 1–12. https://doi.org/10.3389/fevo.2022.833389.
Spagnoletti, FN.; Carmona, M.; Balestrasse, K.; Chiocchio, V.; Giacometti, R.; Lavado, RS. The arbuscular mycorrhizal fungus Rhizophagus intraradices reduces the root rot caused by Fusarium pseudograminearum in wheat. Rhizosphere 2021, 19(100369): 1–8.
Weng, W.; Yan, J.; Zhou, M.; Yao, X.; Gao, A.; Ma, C.; Cheng, J.; Ruan, J. Roles of Arbuscular Mycorrhizal Fungi as a biocontrol agent in the control of plant diseases. Microorganisms 2022, 10(7). https://doi.org/10.3390/microorganisms10071266.
Adeyemi, NO.; Atayese, MO.; Sakariyawo, OS.; Azeez, JO.; Sobowale, SPA.; Olubode, A.; Mudathir, R.; Adebayo, R.; Adeoye, S. Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L.) grown in copper, lead, and zinc contaminated soils. Rhizosphere 2021, 18: 100325. https://doi.org/10.1016/j.rhisph.2021.100325.
Fall, AF.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, SO.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of Arbuscular Mycorrhizal Fungi on soil fertility: contribution in the improvement of physical, chemical, and biological properties of the soil. Front fungal biol 2022, 3: 723892. https://doi.org/10.3389/ffunb.2022.723892.
Halawa, MA.; Halawa, AEA. Reuse treated wastewater in irrigation-review specie of Palm Trees (Pritchardia Beccariana). Int J Water Wastewater Treat 2022, 8(2): 1–6. https://doi.org/10.16966/2381-5299.183.
Klironomos, JN. Variation in plant response to native and exotic Arbuscular mycorrhizal fungi. Ecology 2003, 84 (9): 2292– 2301.
Jones, MD.; Smith, SE. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Can J Bot 2004, 82: 1089–1109. https://doi.org/10.1139/b04-110.
Johnson, NC.; Wilson, GWT.; Bowker, MA.; Wilson, JA.; Miller, RM. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci 2010, 107 (5): 2093–2098. https://doi.org/10.1073/pnas.0906710107.
Smith, FA.; Smith, SE. How useful is the mutualism-parasitism continuum of arbuscular mycorrhizal functioning? Plant Soil 2013, 363(1–2): 7–18. https://doi.org/10.1007/s11104-012-1583-y.
John, SA.; Ray, JG. Optimization of environmental and the other variables in the application of arbuscular mycorrhizal fungi as an ecotechnological tool for sustainable paddy cultivation: a critical review. J Appl Microbiol 2023, 1–24. https://doi.org/10.1093/jambio/lxad111.
St. John, TV. Root size, root hairs, and mycorrhizal infection: a re-examination of Baylis’s hypothesis with tropical trees. New Phytol 1980, 84: 483-487.
Liu, B.; Li, H.; Zhu, B.; Koide, RT.; Eissenstat, DM.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol 2015, 208: 125–136.
Martın-Robles, N.; Lehmann, A.; Seco, E.; Aroca, R.; Rillig, MC.; Milla, R. Impacts of domestication on the arbuscular mycorrhizal symbiosis of 27 crop species. New Phytol 2017, 1–14.
Hui, Z.; Wen-Jing, Z.; Xin-Tao, G.; Ze-Qing, M. Relationships between root hairs and mycorrhizal fungi across typical subtropical tree species. Chin J Plant Ecol 2023, 47(1): 88–100. https://doi.org/10.17521/cjpe.2022.0131.
Broschat, TK. Root and shoot growth patterns in four palm species and their relationships with air and soil temperatures. HortScience 1998, 33(6): 995–998.
Jourdan, C.; Michaux-Ferrière, N.; Perbal, G. Root system architecture and gravitropism in the oil palm. Ann Bot 2000, 85(6): 861–868. https://doi.org/10.1006/anbo.2000.1148.
Safitri, L.; Suryanti, S.; Kautsar, V.; Kurniawan, A.; Santiabudi, F. Study of oil palm root architecture with variation of crop stage and soil type vulnerable to drought. IOP Conf Series: Earth Environ Sci 2018, 141(1): 1–8. https://doi.org/10.1088/1755-1315/141/1/012031.
Amira, JRAD.; Mohamed, BS. Architecture study of the young Date Palm (Phoenix dactylifera L.) root system. J Life Sci 2014, 8(5): 425-432.
Kavadia, A.; Omirou, M.; Fasoula, D.; Trajanoski, S.; Andreou, E.; Loannides, IM. Genotype and soil water availability shapes the composition of AMF communities at chickpea early growth stages. Appl Soil Ecol 2020, 150: 103443. https://doi.org/10.1016/j.apsoil.2019.103443.
Wang, K.; Bi, Y.; Zhang, J.; Ma, S. AMF inoculum enhances crop yields of Zea mays L. ‘Chenghai No. 618’ and Glycine max L. ‘Zhonghuang No. 17’ without disturbing native fungal communities in coal mine dumps. Int J Environ Res Public Health 2022, 19:17058. https://doi.org/10.3390/ijerph192417058.
Badi, OBM.; Abdelhalim, TS.; Eltayeb, MM.; Gorafi, YSA.; Tsujimoto, H.; Taniguchi, T. Dominance of limited arbuscular mycorrhizal fungal generalists of Sorghum bicolor in a semi-arid region in Sudan. Soil Sci Plant Nutr 2019, 65(6): 570–578. https://doi.org/10.1080/00380768.2019.1680573.
Grünfeld, L.; Mola, M.; Wulf, M.; Hempel, S.; Veresoglou, SD. Disentangling the relative importance of spatiotemporal parameters and host specificity in shaping arbuscular mycorrhizal fungus communities in a temperate forest. Mycorrhiza 2021, 31:589–98. https://doi.org/10.1007/s00572-021-01041-6.
Tsiknia, M.; Skiada, V.; Ipsilantis, I.; Vasileiadis, S.; Kavroulakis, N.; Genitsaris, S.; Papadopoulou, KK.; Hart, M.; Klironomos, J.; Karpouzas, DG.; Ehaliotis, C. Strong host-specific selection and over-dominance characterize arbuscular mycorrhizal fungal root colonizers of coastal sand-dune plants of the Mediterranean region. FEMS Microbiol Ecol 2021, 97(9): 1-12. https://doi.org/10.1093/femsec/fia b109
Koch, AM.; Croll, D.; Sanders, IR. Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol Lett 2006, 9:103–10. https://doi.org/10.1111/j.1461-0248.2005.00853.x.
Chaudhary, VB.; O’Dell, TE.; Rillig, MC.; Johnson, NC. Multiscale patterns of arbuscular mycorrhizal fungal abundance and diversity in semiarid shrublands. Fungal Ecol 2014, 12:32–43. https://doi.org/10.1016/j.funeco.2014.06.003.
Koide, RT.; Mosse, B. A history of research on arbuscular mycorrhiza. Mycorrhiza 2004, 14(3): 145–163. https://doi.org/10.1007/s00572-004-0307-4.