Advances in research on the use of Brevundimonas spp. to improve crop and soil fertility and for soil bioremediation
Main Article Content
Abstract
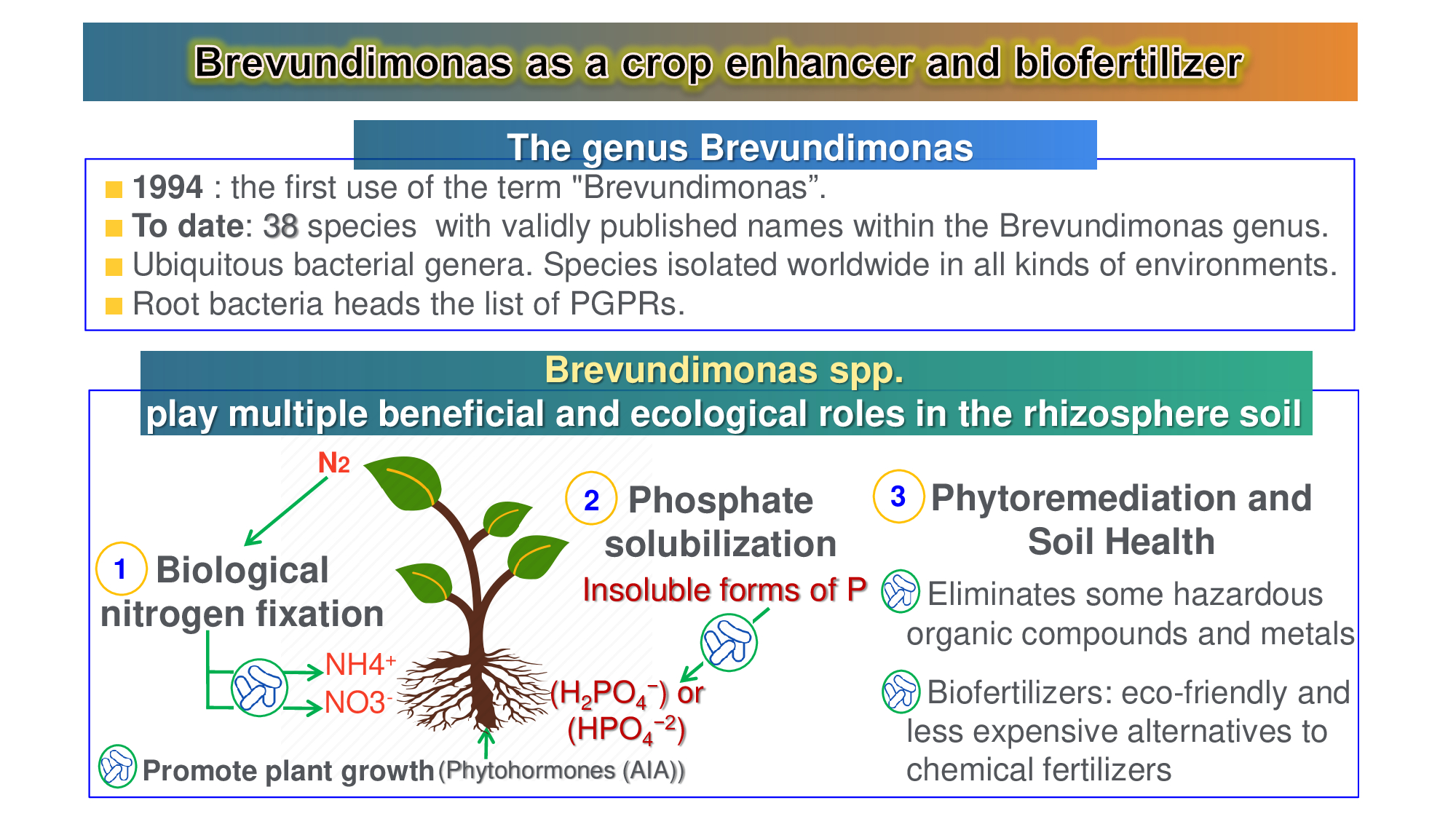
Biofertilizers or biological fertilizers maintain soil fertility by fixing atmospheric nitrogen, solubilizing P and K, producing plant growth substances and antibiotics as well as biodegradation of organic matter in the soil that enriches the root rhizosphere. Microbial biofertilizers are eco-friendly and less expensive alternatives to chemical fertilizers. The key components of healthy soil are populations of plant growth promoting rhizobacteria (PGPR) which play multiple beneficial and ecological roles in the rhizosphere soil. PGPR colonizes rhizosphere or plant roots, resulting in phytostimulation, biofertilization and biocontrol either directly and/or indirectly. Another important role of PGPR is its ability to decontaminate soils through a process called soil bioremediation. Recently, the known rhizobacteria environmentally friendly biofertilizers for sustainable agriculture are those belonging to Brevundimonas spp., which play a significant role in improving crop production and soil health
Article Details
References
Seneviratne G, Jayakody K, Weerasekara MLMAW, Someya T, Ryuda N. Microbial biofertilizer application versus compost use in agriculture: soil health implications. In: Miransari M, editor. Soil microbes and environmental health. New York: Nova Science Publishers, 2011, 4: 81-117.
Javaid A. Arbuscular mycorrhizal mediated nutrition in plants. J Plant Nutr. 2009a , 32(10): 1595-1618. http://dx.doi.org/10.1080/01904160903150875 DOI: https://doi.org/10.1080/01904160903150875
Javaid A. Growth, nodulation and yield of black gram [Vigna mungo (L.) Hepper] as influenced by biofertilizers and soilamendments. Afr J Biotechnol. 2009b, 8(21): 5711-5717. https://doi.org/10.5897/AJB09.793 DOI: https://doi.org/10.5897/AJB09.793
Hazarika BN, Ansari S. Biofertilizers in fruit crops – A review. Agric Rev. 2007, 28(1):69-74.
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv. 2014, 32(2): 429-448. https://doi.org/10.1016/j.biotechadv.2013.12.005 DOI: https://doi.org/10.1016/j.biotechadv.2013.12.005
Javaid A, Ali A, Shoaib A, Khan IH. Alleviating stress of Sclertium rolfsii on growth of chickpea var. Bhakkar-2011 by Trichoderma harzianum and T. viride. J Anim Plant Sci. 2021, 31(6):1755-1761. DOI: https://doi.org/10.36899/JAPS.2021.6.0378
Sharf W, Javaid A, Shoaib A, Khan IH. Induction of resistance in chili against Sclerotium rolfsii by plant growth promoting rhizobacteria and Anagallis arvensis. Egypt J Biol Pest Control. 2021, 31:16. DOI: https://doi.org/10.1186/s41938-021-00364-y
Segers P, Vancanneyt M, Pot B, et al. Classification of Pseudomonas diminuta Leifson and Hugh 1954 and Pseudomonas vesicularis Busing, Doll, and Freytag 1953 in Brevundimonas gen. nov. as Brevundimonas diminuta comb. nov. and Brevundimonas vesicularis comb. nov., respectively. Int J Syst Bacteriol. 1994, 44: 499-510. https://doi.org/10.1099/00207713-44-3-499 DOI: https://doi.org/10.1099/00207713-44-3-499
Abraham WR, Strompl C, Meyer H, et al. Phylogeny and polyphasic taxonomy of Caulobacter species. Proposal of Maricaulis gen. nov. with Maricaulis maris (Poindexter) comb. nov. as the type species, and emended description of the genera Brevundimonas and Caulobacter. Int J Syst Bacteriol. 1999, 49: 1053-1073. https://doi.org/10.1099/00207713-49-3-1053 DOI: https://doi.org/10.1099/00207713-49-3-1053
Dahal RH, Kim J. Brevundimonas humi sp. nov., an alphaproteobacterium isolated from forest soil. Int J Syst Evol Microbiol. 2018, 68: 709-714. https://doi.org/10.1099/ijsem.0.002559 DOI: https://doi.org/10.1099/ijsem.0.002559
Kang SJ, Choi NS, Choi JH, Lee JS, Yoon JH, Song JJ. Brevundimonas naejangsanensis sp. nov., a proteolytic bacterium isolated from soil, and reclassification of Mycoplana bullata into the genus Brevundimonas as Brevundimonas bullata comb. nov. Int J Syst Evol Microbiol. 2009, 59: 3155-3160. https://doi.org/10.1099/ijs.0.011700-0 DOI: https://doi.org/10.1099/ijs.0.011700-0
Tsubouchi T, Koyama S, Mori K, et al. Brevundimonas denitrificans sp. nov., a denitrifying bacterium isolated from deep subseafloor sediment. Int J Syst Evol Microbiol. 2014, 64: 3709-3716. https://doi.org/10.1099/ijs.0.067199-0 DOI: https://doi.org/10.1099/ijs.0.067199-0
Peng M, Zhao Z, Liang Z. Biodegradation of ochratoxin A and ochratoxin B by Brevundimonas naejangsanensis isolated from soil. Food Control. 2022, 133: 108611. https://doi.org/10.1016/j.foodcont.2021.108611 DOI: https://doi.org/10.1016/j.foodcont.2021.108611
Lee YW, Lee KH, Lee SY, Im W T. Brevundimonas fluminis sp. nov., isolated from a river. Int J Syst Evol Microbiol. 2020, 70(1): 204-210. https://doi.org/10.1099/ijsem.0.003736 DOI: https://doi.org/10.1099/ijsem.0.003736
Kumar V, Gera R. Isolation of a multi-trait plant growth promoting Brevundimonas sp. and its effect on the growth of Bt-cotton. 3 Biotech. 2014, 4: 97-101. https://doi.org/10.1007/s13205-013-0126-4 DOI: https://doi.org/10.1007/s13205-013-0126-4
Menéndez E, Pérez-Yepes J, Carro L, Fernández-Pascual M, Ramírez-Bahena MH, Klenk HP, Barrios ML, Peix A, Velázquez E. Brevundimonas canariensis sp. nov., isolated from roots of Triticum aestivum. Int J Syst Evol Microbiol. 2017 67(4): 969-973. https://doi.org/10.1099/ijsem.0.001725 DOI: https://doi.org/10.1099/ijsem.0.001725
Rathi M, Yogalakshmi KN. Brevundimonas diminuta MYS6 associated Helianthus annuus L. for enhanced copper phytoremediation. Chemosphere, 2021, 263:128195. https://doi.org/10.1016/j.chemosphere.2020.128195 DOI: https://doi.org/10.1016/j.chemosphere.2020.128195
Wang C, Zhang M, Cheng F, Geng Q. Biodegradation characterization and immobilized strains’ potential for quinoline degradation by Brevundimonas sp. K4 isolated from activated sludge of coking wastewater. Biosci Biotechnol Biochem. 2015, 79: 164–170. https://doi.org/10.1080/09168451.2014.952615 DOI: https://doi.org/10.1080/09168451.2014.952615
Naqqash T, Imran A, Hameed S, et al. First report of diazotrophic Brevundimonas spp. as growth enhancer and root colonizer of potato. Sci Rep. 2020, 10: 12893. https://doi.org/10.1038/s41598-020-69782-6 DOI: https://doi.org/10.1038/s41598-020-69782-6
Zaim S, Bekkar AA, Belabid L. 2017 Rhizobium as a Crop Enhancer and Biofertilizer for Increased Non-legume Production. In: Hansen A, Choudhary D, Agrawal P, Varma A, editors. Rhizobium Biology and Biotechnology. Cham: Springer, Soil Biology, vol 50, 2017, 25-37. https://doi.org/10.1007/978-3-319-64982-5_3 DOI: https://doi.org/10.1007/978-3-319-64982-5_3
Saikia SP, Vanita J. Biological nitrogen fixation with non-legumes: an achievable target or a dogma?. Curr Sci. 2007, 92(3): 317-322.
dos Santos SG, da Silva Ribeiro F, Alves GC, Santos LA, Reis VM. Inoculation with five diazotrophs alters nitrogen metabolism during the initial growth of sugarcane varieties with contrasting responses to added nitrogen. Plant Soil. 2020, 44: 363–370. https://doi.org/10.1007/s11104-019-04101-1 DOI: https://doi.org/10.1007/s11104-019-04101-1
Jesus ED, Leite RD, Bastos RDA, Aragão OODS, Araújo AP. Co-inoculation of Bradyrhizobium stimulates the symbiosis efficiency of Rhizobium with common bean. Plant Soil. 2018, 425: 201-215. https://doi.org/10.1007/s11104-017-3541-1 DOI: https://doi.org/10.1007/s11104-017-3541-1
Li HB, Singh RK, Singh P, Song QQ, Xing YX, Yang LT, Li YR. Genetic Diversity of Nitrogen-Fixing and Plant Growth Promoting Pseudomonas Species Isolated from Sugarcane Rhizosphere. Front Microbiol. 2017, 8: 1268. https://doi.org/10.3389/fmicb.2017.01268 DOI: https://doi.org/10.3389/fmicb.2017.01268
Singh R, Singh P, Haibi L, et al. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: a comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20: 220. https://doi.org/10.1186/s12870-020-02400-9 DOI: https://doi.org/10.1186/s12870-020-02400-9
Szilagyi-Zecchin VJ, Ikeda AC, Hungria M, Adamoski D, Kava-Cordeiro V, Glienke C, Galli-Terasawa LV. Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Exp. 2014, 4: 26. https://doi.org/10.1186/s13568-014-0026-y DOI: https://doi.org/10.1186/s13568-014-0026-y
Brusamarello-Santos LCC, Pacheco F, Aljanabi SMM, et al. Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae. Plant Soil. 2012, 356(1): 113-125. https://doi.org/10.1007/s11104-011-1044-z DOI: https://doi.org/10.1007/s11104-011-1044-z
Zaim S, Belabid L, Bayaa B, Bekkar AA. Biological control of chickpea Fusarium wilts using rhizobacteria “PGPR”. In: Choudhary D, Varma A, editors. Microbial-mediated Induced Systemic Resistance in Plants. Singapore: Springer; 2016, 147-162. https://doi.org/10.1007/978-981-10-0388-2_10 DOI: https://doi.org/10.1007/978-981-10-0388-2_10
Rana A, Saharan B, Joshi M, Prasanna R, Kumar K, Nain L. Identification of multi- trait PGPR isolates and evaluating their potential as inoculants for wheat. Ann Microbiol. 2011, 61: 893–900. https://doi.org/10.1007/s13213-011-0211-z DOI: https://doi.org/10.1007/s13213-011-0211-z
Gopalakrishnan S, Srinivas V, Saminen S. Nitrogen fixation, plant growth and yield enhancements by diazotrophic growth-promoting bacteria in two cultivars of chickpea (Cicer arietinum L.). Biocatal Agric Biotechnol. 2017, 11: 116-123. https://doi.org/10.1016/j.bcab.2017.06.012 DOI: https://doi.org/10.1016/j.bcab.2017.06.012
dos Santos SG, da Silva Ribeiro F, Fonseca CS, Pereia W, Santos LA, Reis VM. Development and nitrate reductase activity of sugarcane inoculated with five diazotrophic strains. Arch Microbiol. 2017, 199: 863-873. https://doi.org/10.1007/s00203-017-1357-2 DOI: https://doi.org/10.1007/s00203-017-1357-2
Li JH, Wang ET, Chen WF, Chen WX. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem. 2008, 40(1): 238-246. https://doi.org/10.1016/j.soilbio.2007.08.014 DOI: https://doi.org/10.1016/j.soilbio.2007.08.014
Sharma P, Chaturvedi P, Chandra R, Kumar S. Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere 2022, 295: 133823. https://doi.org/10.1016/j.chemosphere.2022.133823 DOI: https://doi.org/10.1016/j.chemosphere.2022.133823
Soto J, Charles TC, Lynch MDJ, Larama G, Herrera H, Arriagada C. Genome sequence of Brevundimonas sp., an arsenic resistant soil bacterium. Diversity. 2021, 13(8): 344. https://doi.org/10.3390/d13080344 DOI: https://doi.org/10.3390/d13080344
Park Y, Je KW, Lee K, Jung SE, Choi TJ. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia. 2008, 598: 219–228. https://doi.org/10.1007/s10750-007-9152-8 DOI: https://doi.org/10.1007/s10750-007-9152-8
Richardson AE, Simpson RJ. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156(3): 989-996. https://doi.org/10.1104/pp.111.175448 DOI: https://doi.org/10.1104/pp.111.175448
Bagyaraj DJ, Sharma MP, Maiti D. Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr Sci. 2015, 108(7): 1288-1293.
Rashid M, Khalil S, Ayub N, Alam S, Latif F. Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pak J Biol Sci. 2004, 7(2): 187-196. https://doi.org/10.3923/pjbs.2004.187.196 DOI: https://doi.org/10.3923/pjbs.2004.187.196
Gull M, Hafeez FY, Saleem M, Malik KA. Phosphorus uptake and growth promotion of chickpea by co-inoculation of mineral phosphate solubilising bacteria and a mixed rhizobial culture. Aust J Exp Agric. 2004, 44: 623-628. https://doi.org/10.1071/EA02218 DOI: https://doi.org/10.1071/EA02218
Cheng D, Tian Z, Feng L, Xu L, Wang H. Diversity analysis of the rhizospheric and endophytic bacterial communities of Senecio vulgaris L. (Asteraceae) in an invasive range. PeerJ. 2019, 6: e6162. https://doi.org/10.7717/peerj.6162 DOI: https://doi.org/10.7717/peerj.6162
Liaqat F, Eltem R. Identification and characterization of endophytic bacteria isolated from in vitro cultures of peach and pear rootstocks. 3 Biotech. 2016, 6: 120. https://doi.org/10.1007/s13205-016-0442-6 DOI: https://doi.org/10.1007/s13205-016-0442-6
Singh N, Marwa N, Mishra J, Verma PC, Rathaur S, Singh N. Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol Environ Saf. 2016, 125: 25-34. https://doi.org/10.1016/j.ecoenv.2015.11.020 DOI: https://doi.org/10.1016/j.ecoenv.2015.11.020
Pastore M, Sforza E. Exploiting symbiotic interactions between Chlorella protothecoides and Brevundimonas diminuta for an efficient single-step urban wastewater treatment. Water Sci Technol. 2018, 78(1):216-224. https://doi.org/10.2166/wst.2018.155 DOI: https://doi.org/10.2166/wst.2018.155
Doran JW, Zeiss MR. Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol. 2000, 15: 3–11. https://doi.org/10.1016/S0929-1393(00)00067-6 DOI: https://doi.org/10.1016/S0929-1393(00)00067-6
Han Q, Wang M, Cao J, et al. Health risk assessment and bioaccessibilities of heavy metals for children in soil and dust from urban parks and schools of Jiaozuo, China. Ecotoxicol Environ Saf. 2020, 191: 110157. https://doi.org/10.1016/j.ecoenv.2019.110157 DOI: https://doi.org/10.1016/j.ecoenv.2019.110157
Pulleman M, Creamer R, Hamer U, Helder J, Pelosi C, Pérès G, Rutgers M. Soil biodiversity, biological indicators and soil ecosystem services-an overview of European approaches. Curr Res Environ Sustain. 2012 4(5): 529-538. https://doi.org/10.1016/j.cosust.2012.10.009 DOI: https://doi.org/10.1016/j.cosust.2012.10.009
Ruley JA, Tumuhairwe JB, Amoding A, Westengen OT, Vinje H. Rhizobacteria communities of phytoremediation plant species in petroleum hydrocarbon contaminated soil of the Sudd ecosystem, South Sudan. Int J Microbiol. 2020, 2020. https://doi.org/10.1155/2020/6639118 DOI: https://doi.org/10.1155/2020/6639118
Kong Z, Glick BR. The role of plant growth-promoting bacteria in metal phytoremediation. Adv Microb Physiol. 2017, 71: 97-132. https://doi.org/10.1016/bs.ampbs.2017.04.001 DOI: https://doi.org/10.1016/bs.ampbs.2017.04.001
Sharma P, Tripathi S, Chandra R. Phytoremediation potential of heavy metal accumulator plants for waste management in the pulp and paper industry. Heliyon, 2020, 6(7): e04559. https://doi.org/10.1016/j.heliyon.2020.e04559 DOI: https://doi.org/10.1016/j.heliyon.2020.e04559
Wu CH, Wood TK, Mulchandani A, Chen W. Engineering plant-microbe symbiosis for rhizoremediation of heavy metals. Appl Environ Microbiol. 2006, 72(2): 1129-1134. https://doi.org/10.1128/AEM.72.2.1129-1134.2006 DOI: https://doi.org/10.1128/AEM.72.2.1129-1134.2006
Khan S, Farooq R, Shahbaz S, Khan MA, Sadique M. Health risk assessment of heavy metals for population via consumption of vegetables. World Appl Sci J. 2009, 6(12): 1602-1606.
Saier MH. Beneficial bacteria and bioremediation. Water Air Soil Pollut. 2007, 184(1): 1-3. https://doi.org/10.1007/s11270-006-2431-6 DOI: https://doi.org/10.1007/s11270-006-2431-6
Sharma P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: an update. Bioresour Technol. 2021, 328: 124835. https://doi.org/10.1016/j.biortech.2021.124835 DOI: https://doi.org/10.1016/j.biortech.2021.124835
Singh N, Gadi R. Studies on biosorption of Pb (II) by the nonliving biomasses of Pseudomonas oleovorans and Brevundimonas vesicularis and its removal from wastewater samples. Eur J Sci Res. 2012, 69(2): 279-289.
Irawati W, Soraya Y, Baskoro AH. A study on mercury-resistant bacteria isolated from a gold mine in Pongkor village, Bogor, Indonesia. Hayati Journal of Biosciences. 2012, 19(4): 197-200. https://doi.org/10.4308/hjb.19.4.197 DOI: https://doi.org/10.4308/hjb.19.4.197
Zhang Ch, Li J, An H, Wu X, Wu Y, Long Y, Li R, Xing D. Enhanced elimination of dimethachlon from soils using a novel strain Brevundimonas naejangsanensis J3. J Environ Manage. 2019, 255: 109848. https://doi.org/10.1016/j.jenvman.2019.109848 DOI: https://doi.org/10.1016/j.jenvman.2019.109848
Singh S, Kumar V, Gupta P, Singh A. Conjoint application of novel bacterial isolates on dynamic changes inoxidative stress responses of axenic Brassica juncea L. in Hg-stress soils. J Hazard Mater. 2022, 434: 128854. https://doi.org/10.1016/j.jhazmat.2022.128854 DOI: https://doi.org/10.1016/j.jhazmat.2022.128854