Application of polyvinylpyrrolidone-iodine complex as corrosion inhibitor for carbon steel using an experimental design method
DOI:
https://doi.org/10.57056/ajet.v6i1.68Keywords:
Corrosion, Carbon Steel, Polyvinylpyrrolidone-iodine complex, Inhibition efficiency, Design of experimentAbstract
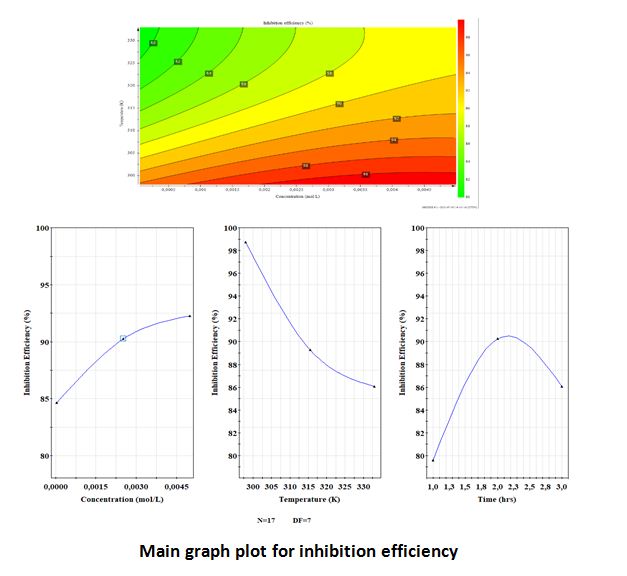
Corrosion processes are responsible for numerous losses, especially in the industrial sector. Inhibitors are commonly used to prevent corrosion in acidic medium. The aim of this study was to apply an experimental design to optimize the influencing parameters such as inhibitor concentration, temperature and immersion time on the corrosion inhibition of polyvinylpyrrolidone-iodine (PVP-I) complexes on carbon steel using the weight loss technique (WL). The parameters of the corrosion protection process were optimized and predictive mathematical models were developed using the Response Surface Methodology (RSM) using the Central Composite Design (CCD). It was also found that the data predicted by the regression analysis had a good agreement with the data obtained from the experiments, with the values R2 = 0.999 and Adj. R2 = 0.997 for the inhibitory effect. The best efficiencies for experiments that were not performed were determined by experimental design (DOE)
References
Abd El-Maksoud SA, Fouda A. Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Materials Chemistry and Physics. 2005, 93:84-90
Attar T, Benchadli A, Choukchou-Braham E. Corrosion inhibition of carbon steel in perchloric acid by potassium iodide. Int J Adv Chem. 2019, 7: 35–41
Ikumapayi CM, Adeniji AA, Idehen EO, Barnabas AA. Combating chloride ions in reinforced concrete using K2Cr2O7 as corrosion inhibitor. Alg. J. Eng. Tech. 2020, 3: 064-068
Evrim B, Ahmet C, Birgu Y. Inhibitory effect of Gentiana olivEIri extracts on the corrosion of mild steel in 0.5 M HCl: Electrochemical and phytochemical evaluation. Arabian Journal of Chemistry. 2019, 12:4303–4319.
Attar T, Benchadli A, Messaoudi B, Benhadria N, Choukchou-Braham E. Experimental and Theoretical Studies of Eosin Y Dye as Corrosion Inhibitors for Carbon Steel in Perchloric Acid Solution. Chem React Eng Catal. 2020, 15:454–464.
Bouraoui MM, Chettouh S, Chouchane T, Khellaf N. Inhibition Efficiency of cinnamon oil as a green corrosion inhibitor. Journal of Bio and Tribo-Corrosion. 2019, 5: 1–9
Attar T, Larabi L, Harek Y. Corrosion inhibition of cold rolled steel in 0.5 M H2SO4 by potassium iodide. Der Pharma Chemica. 2014, 6:181–186.
Husaini M, Usman B, Ibrahim MB. Study of corrosion inhibition performance of Glutaraldehyde on Aluminium in nitric acid solution. Alg. J. Eng. Tech. 2020: 2:003-010.
Ostapenko GI, Gloukhov PA, Bunev AS. Document investigation of 2-cyclohexenylcycl-ohexanone as steel corrosion inhibitor and surfactant in hydrochloric acid. Corros. Sci. 2014, 82: 265-270.
Popova A, Christov M, Vasilev A. Mono- and dicationic benzothiazolic quaternary ammonium bromides as mild steel corrosion inhibitors. Part III: influence of the temperature on the inhibition process. Corros. Sci. 2015, 94: 70-78.
Jones DS, Djokic J, Gorman SP. The resistance of polyvinylpyrro-lidone-iodine-poly(caprolactone) blends to adherence of Escherichia coli. Biomaterials. 2005, 26; 2013–2020.
Benchadli A, Attar T, Messaoudi B, Choukchou-Braham E. Polyvinylpyrrolidone as a corrosion inhibitor for carbon steel in a perchloric acid solution: effect of structural size. Hungarian Journal of Industry and Chemistry. 2021, 49: 59–69.
Femiana Gapsari WS, Soenoko R, Suprapto A. Minimization of corrosion rate using response surface methodology. Eng Rev. 2018, 38:115–9.
Prabhua PR, Deepa P, Padmalatha R. Analysis of Garcinia indica Choisy extract as eco-friendly corrosion inhibitor for aluminum in phosphoric acid using the design of experiment. Journal of Materials Research and Technology. 2020, 9: 3622-3631.
Attar T, Benchadli A, Mellal T, Dali Youcef B and Choukchou-Braham E. Use of Experimental Designs to Evaluate the Influence of Methyl Green Dye as a Corrosion Inhibitor for Carbon Steel in Perchloric Acid. M J Chem. 2021, 23: 60-69.
Nwose SA, Edoziuno FO, Osuji SO. Statistical analysis and Response Surface Modelling of the compressive strength inhibition of crude oil in concrete test cubes. Alg. J. Eng. Tech. 2021, 4:99-107.
Afzalkhah M, Masoum S, Behpour M, Naeimi H, Reisi-Vanani A. Experimental and Theoretical Investigation of Inhibition Efficiency of 2-(2-Hydroxyphenyl)- benzothiazole Using Impedance Spectroscopy, Experimental Design, and Quantum Chemical Calculations. Ind. Eng. Chem. Res. 2017, 56: 9035– 9044.
Maher AA, Al-Shamkhani T. Use of experimental designs to evaluate the influence of Ziziphus Leaves extracts as a corrosion inhibitor for mild steel in (3.5M) NaCl. Int J Appl Eng Res Dev. 2018, 13:7416–23.
Attar T, Nouali F, Kibou Z, Benchadli A, Messaoudi B. Corrosion inhibition, adsorption and thermodynamic properties of 2-aminopyridine derivatives on the corrosion of carbon steel in sulfuric acid solution. J. Chem. Sci. 2021, 133: 1-10.
Benchadli A, Attar T, Choukchou-Braham E. Corrosion inhibition of carbon steel (XC 38) in hydrochloric acid by potassium iodide. Journal of Advanced Research in Science and Technology. 2018, 5: 834–844.
Gao XL, Dai K, Wang Z, Wang TT, He JB, Dai K. Establishing quantitative structure tribo-ability relationship model using Bayesian regularization neural network. Friction. 2016, 4: 105–115.
Eddy NO, Odoemelam SA, Odiongenyi AO. Inhibitive, adsorption and synergistic studies on ethanol extract of Gnetum africana as green corrosion inhibitor for mild steel in H2SO4. Green. Chem. Lett. Rev. 2009, 2: 111–119.
Benchadli A, Attar T, Choukchou-Braham E. Inhibition of Carbon Steel Corrosion in Perchloric Acid Solution by Povidone Iodine. Phys. Chem. Res. 2019, 7: 837–848.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Tarik Attar, Esma Choukchou-Braham, Boumediene Dali Youcef, Abbes Benchadli, Tayeb Mellal, Ilyes Benabdelkader

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





