Study of the elaboration of U3O8 by the Modified Direct Denitration process (MDD)
DOI:
https://doi.org/10.57056/ajet.v8i2.129Keywords:
Modified direct denitration process, Uranyl Nitrate (UNH), Uranium trioxide, Triuranium octoxideAbstract
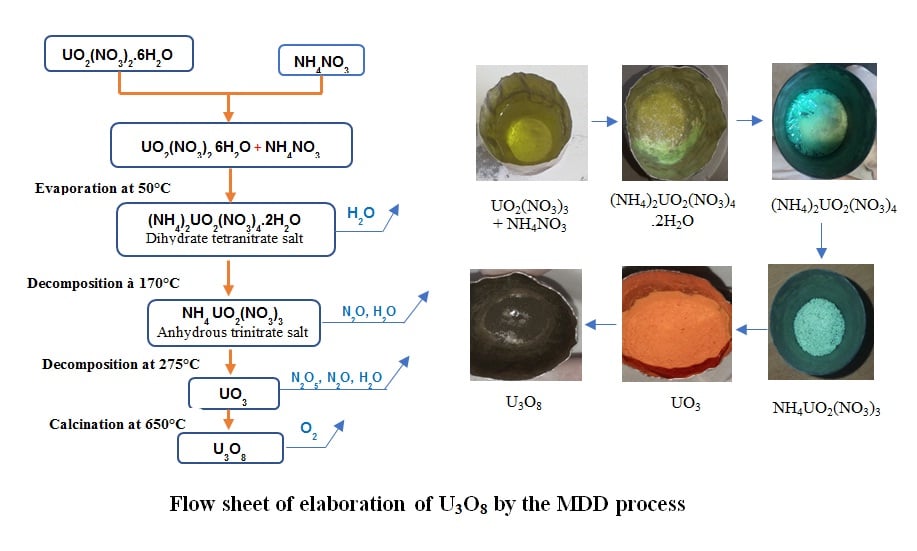
In the nuclear industry, uranium trioxide (UO3) is considered an intermediate in the preparation of uranium metal or uranium dioxide (UO2), which are the two most widely used nuclear fuels. Several processes have been described for transforming solutions of uranyl nitrate UO2(NO3)2 into uranium trioxide. Among these processes, Modified Direct Denitration (MDD) has been implemented to prepare Uranium trioxide with high reactivity. This process consists of adding ammonium nitrate to a pure uranyl nitrate solution to form the double salt (NH4)2UO2(NO3)4.2H2O which decomposes by calcination to produce a UO3 powder. The objective of this work is to study, first, the thermal decomposition of double salt (NH4)2UO2(NO3)4.2H2O in nitrogen atmosphere by thermogravimetry in order to determine the formation temperatures of the different phases and second, the determination of the optimal parameters (time and temperature) to prepare stable triuranium octoxide (U3O8) using muffle furnace. As results, the MDD product obtained is an orange colored and free flowing UO3 powder, having a surface area in the target range [5-12 m2/g]. In addition, by calcination of UO3 powder at 650°C for one hour, U3O8 oxide is obtained. The identification of the latter by the O/U ratio gave a value of 2.65, which is in the range [2.6-2.66]. This suggests that the oxide produced under these conditions is β-U3O8.
References
Ondrcjcin RS, Garrctt Jr TP. The thermal decomposition of anhydrous uranyl nitrate and uranyl nitrate dihydrate. Journal of Physical Chemistry. 1961; 65(3): 470-473.
Hartland S, Ncsbitt RJ. Thermal decomposition of uranyl nitrate hexahydrate. Journal of Applied Chemistry. 1965; 14 (9): 406-412.
Lodding W, Ojamaa L. Dehydration and thermal decomposition of uranyl nitrates in the presence of steam, Journal of Inorganic and Nuclear Chemistry, 1965; 27 (6): 1261-1268.
Smith WH. Thermal dehydration of uranyl nitrate hydrates. Journal of Inorganic and Nuclear Chemistry. 1968; 30 (7): 1761-1768.
Bakel AJ, Quigle KJ, Vandegrift GF. Argonne National Laboratory (ANL) Progress in Minimizing Effects of LEU Conversion on Calcination of Fission Product 99Mo Acid Waste Solution. Proceedings of RERTR, International Meeting, San Carlos de Bariloche, Argentina. 2002.
Collins E. D. Personal communication from Collins (Oak Ridge National Laboratory) to Bakel A. (Argonne National Laboratory), 2013, 16 February 2013.
Slagle OD, Davis NC, Parehen LJ. AVLIS Modified Direct Denitration: UO3 Powder Evaluation, PNL-848, Pacific Northwest Laboratory.1993 : January 1993.
Florence TM. A review and comparison of methods fort the determination of Oxygen/Uranium ratios in uranium oxides. Analytical methods in the nuclear fuel cycle IAEA,Vienne 1972. AIEA. SM.149/64, 45. Australian Atomic Energy Commission research establishement, Lucas Heights, Australia. 1972.
Bakel AJ, George F, Vandegrift GF, Quigley KJ., Aase SB, Neylon MK, Carney KP, Travelli A. ANL Progress in Minimizing Effects of LEU Conversion on Calcination of Fission-Product 99Mo Acid Waste Solution. 25th International Meeting on Reduced Enrichment for Research and Test Reactors. Chicago IL, USA. 2003, October 5-10, 2003.
Kitts FG. Pilot Scale demonstration of the Modified Direct Denitration Process to Prepare Uranium Oxide for Fuel Fabrication Evaluation. Oak Ridge National Laboratory, ORNL/TM-12726, 1994.
Felker LK, Vedder RJ, Walker EA, Collins ED. Product Conversion: The Link between Separations and Fuel Fabrication, ATALANTE. 2008.
Paul A, Haas Ray D, Arthur W, Stines, B. Development of Thermal Denitration to Prepare Uranium Oxide and Mixed Oxides for Nuclear Fuel Fabrication. 1981, September 1981.
Notz KJ, Haas PA. Properties and Thermal Decomposition of the Double Salts of Uranyl Nitrate Ammonium Nitrate. Oak Ridge National Laboratory, ORNL/TM-7820. 1981.
Hoekstra HR, Siegel S. The uranium-oxygen system: U3O8-UO3. Journal of Inorganic and Nuclear Chemistry. 1961, 18: 154-165.
Thomas R. Réactivation des oxydes d’uranium en vue de leur hydrofluoration : influence des additifs et mécanismes. Thèse de Rudy Thomas, Lille 1, 2011.
Lister BAJ, Richardson RJ. The preparation of uranium trioxide by thermal decomposition of uranyl nitrate. A.E.R.E. C/R 2276. 1954, October 1954.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Messaoud Bennemla, Toufik Semaoune, Yasmina Hammache, Sihem Ouatas, Fatima Lekouara, Meriem Chabane Sari, Dallal Chebouki

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





