Gamma spectrometry technique application to the 60Co sorption onto IRN-77 resin from radioactive wastewater: Equilibrium, Kinetic and Thermodynamic investigations
DOI:
https://doi.org/10.57056/ajet.v8i2.122Keywords:
Nuclear wastewater, 60Co, kinetic study, Adsorption, Gamma spectrometry, IRN-77Abstract
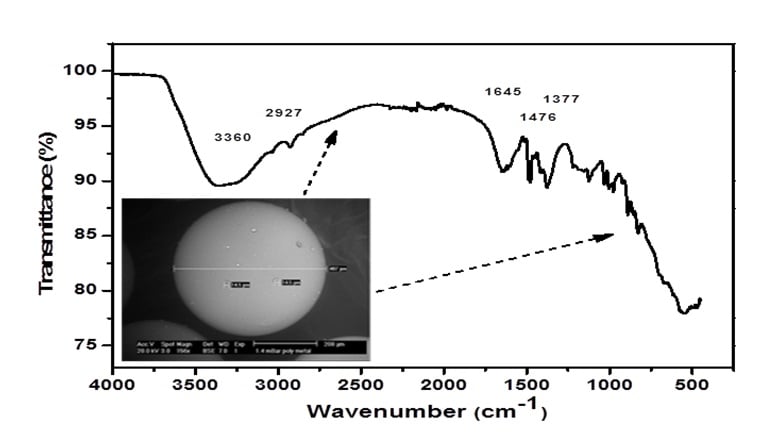
The performance of synthetic ion exchange resin IRN-77 have been studied in this work in order to use it as an adsorbent to remove radioactive isotope 60Co from nuclear wastewater by the sorption process, using the gamma spectrometry technique. The resin simple was identified using SEM and FTIR infrared spectrometry. The gamma radiation acquisition emitted from the fixed radioactive 60Co onto IRN-77 solid samples was carried out using the gamma spectrometry chain, equipped with an HPGe semi-conductor detector with high-resolution. Various factors were considered for the sorption process study such as 60Co concentration, contact time and temperature. The maximum adsorption capacity of the IRN-77 samples was determined by studying the adsorption isotherms; Kinetics models including thermodynamics were also studied and investigated. The experimental results showed that the adsorption reaction was adjustable to the pseudo-first-order and the Langmuir model was found to describe best the experimental results by obtaining a very important maximum adsorption quantity of 10.620 µCi of 60Co per 1 gram of IRN-77 adsorbent. A dimensionless separation factor RL was used to judge the favorable adsorption. The adsorption capacity of 60Co ions onto IRN-77 particles increased with the increasing of temperature. The values of the thermodynamic parameters have shown that the 60Co ions adsorption process was endothermic and favored at high temperatures with a positive value of the enthalpy ∆H° of 23,54 kJ/mol. The free energy’s values ∆G◦ are positive over the whole temperature range. The specific activities of the fixed 60Co radionuclide allow evaluating the solid samples IRN-77 resin's sorption capacity.
References
Buchtela K. Radiochemical Methods Overview. Elsevier, (2019): 23-30.
Achour S, Amokrane S, Chegrouche S. et al. Adsorption Mechanism Study of Radionuclide 60Co by Purified and α-Fe2O3-Supported Bentonite from Radioactive Solution. Arab J Sci Eng. 2021. https://doi.org/10.1007/s13369-021-05570-2
Omar H, Arida H, Daifullah A. Adsorption of 60Co radionuclides from aqueous solution by raw and modified bentonite. Appl. Clay Sci. 2009; 44: 21–26.
Liang Chen, Shaoming Yu, Liming Zuo, Bin Liu, Lingli Huang. Investigation of Co (II) sorption on GMZ bentonite from aqueous solutions by batch experiments.J.RadioanalNucl Chem.2011; 289:511–520. https://doi.org/10.1007/s10967-011-1098-7
Manohar DM, Noeline BF, Anirudhan TS. Adsorption performance of Al-pillared bentonite clay for the removal of cobalt (II) from aqueous phase. Appl Clay Sci. 2006;31:194–206.
Shao DD, Fan QH, Li JX, Niu ZW, Wu WS, Chen YX, Wang XK. Microporous Mesopororous Mater. 2009;123:1–9.
Tan XL, Fang M, Li JX, Lu Y, Wang XK. Adsorption of Eu (III) onto TiO2: effect of pH, concentration, ionic strength and soil fulvic acid. J Hazard Mater. 2009;168:458–465.
Achour S, Amokrane S, Chegrouche S, Nibou D, Baaloudj O. Artifcial neural network modeling of the hexavalent uranium sorption onto chemically activated bentonite. Research on Chemical Intermediates.2021. https://doi.org/10.1007/s11164-021-04541-4.
Yang A, Wang Z, Zhu Y, Sci. Rep. 2020, 10: 1.
Abdi S, Nasiri M, Mesbahi A, Khani MH. Investigation of uranium (VI) adsorption by polypyrrole. Journal of Hazardous Materials. 2017;332:132-139.
Barkat M, Nibou D, Amokrane S, Chegrouche S, Mellah A. Uranium (VI) adsorption on synthesized 4A and P1 zeolites: equilibrium, kinetic, and thermodynamic studies. Comptes Rendus Chimie. 2015;18(3):261-269.
Pandey S, Fosso-Kankeu E, Redelinghuys J, Kim J, Kang M. Implication of biofilms in the sustainability of acid mine drainage and metal dispersion near coal tailings. Science of the total environment. 2021;788:147851.
Metwally SS, Ayoub RR. Modification of natural bentonite using a chelating agent for sorption of 60Co radionuclide from aqueous solution.Appl. Clay Sci. 2016;126:33-40.
Kundari NA, Permadi MG, Megasari K, Nurliati G. Adsorption of Cobalt-60 (II) on silica xerogel from rice husk. InJournal of Physics: Conference Series 2019 Sep 1 (Vol. 1295, No. 1, p. 012038). IOP Publishing.
Aşçı Y, Kaya, ŞEFİKA. Sorption of cobalt (II) from an aqueous medium using Amberlite 200C and Dowex 88 resins: Equilibrium and kinetic studies. Desalination and Water Treatment. 2016; 57(28): 13091-13105.
Kabak B, Trak D, Kenduzler E, Tomul F, Arslan Y. Separation and preconcentration of cobalt (II) from water samples with Amberlite CG-120 resin. Iranian Journal of Chemistry and Chemical Engineering. 2020; 39(5): 181-189.
Ahmad A, Siddique JA, Laskar MA, Kumar R, Mohd-Setapar SH, Khatoon A, Shiekh RA. New generation Amberlite XAD resin for the removal of metal ions: A review. Journal of Environmental Sciences. 2015; 31:104-123.
Achour S, Azbouche A, Amokrane S, Chegrouche, S. Characterization Study of Algerian Bentonite Samples Using Nuclear Techniques Analysis for Environment Applications. In: 2nd National Conference on Computational Fluid Dynamics & Technology. 2018.
Achour S. Valorisation d’adsorbant pour l’élimination de certains polluants industriels et nucléaires. Phd Thesis, USTHB, Algiers, Algeria Order n°: 01/2023-D/GP. 2023.
Ngah W,W Saime, Ariff NFM, Hashim A, Hanafiah M.A.K.M. Malachite green adsorption onto chitosan coated bentonite beads: isotherms, kinetics and mechanism. Clean Soil, Air, Water.2010; 38: 394–400.
Giles C H, MacEwan T H, Nakhwa SN, SmithD. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. Journal of the Chemical Society (Resumed). 1960; 786: 3973-3993.
Daou TJ. Performance des Nano-sponges de zéolithe de type BEA modifiées par un tensioactif pour l'élimination du nitrate dans l'eau contaminée: effet de la surface externe. Journal des Matériaux Dangereux. 2018.
Benmessaoud A, Nibou D, Mekatel EH, Amokrane S. A comparative study of the linear and non-linear methods for determination of the optimum equilibrium isotherm for adsorption of Pb2+ ions onto Algerian treated clay. Iran. J. Chem. Chem. Eng. 2020; 39 (4): 153-171.
Houhoune F, Nibou D Amokrane S, Barkat M. Modelling and adsorption studies of removal uranium (VI) ions on synthesised zeolite NaY. Des. Water. Treat. 2013,51(28-30): 5583-5591.
Lamgmuir I. The constitution and fundamental properties of solids and liquids, part 1. Solids. J. Am. Chem. Soc. 1916, 38:2221–2295.
Freundlich H.Uber die adsorption in losungen [Adsorption in solution]. Z. Phys. Chem. 1906; 57: 385-470.
Miaoying He, Yi Zhu, Yang Yang, Boping Han, Yuanming Zhang, Adsorption of cobalt(II) ions from aqueous solutions by palygorskite, Applied Clay Science. 2011; 54(3–4): 292-296, https://doi.org/10.1016/j.clay.2011.09.013.
Bhattacharya AK, Naiya TK, Mandal SN, Das SK. Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents”,Chemical Engineering Journal.2008; 137(3) : 529-541.
Abou-Mesalam MM.Applications of Inorganic Ion Exchangers: II-Adsorption of some heavy metal ions from their aqueous waste solution using synthetic iron III titanate. Adsorption. 2004; 10: 87-92.
Nibou D.Mekatel, H.;Amokrane, S.; Barkat, M.;Trari, M.:Adsorption of Zn2+ ions onto NaA and NaX zeolites: Kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 2010; 173: 637-646.
Li XL, Chen CL, Chang PP, Yu SM,Wu WS,Wang XK. Comparative studies of cobalt sorption and desorption on bentonite, alumina and silica: effect of pH and fulvic acid. Desalination.2009, 244:283–292.
Ferhat D, Nibou D, Mekatel EH, Amokrane S. Adsorption of Ni2+ ions onto NaX and NaY zeolites: Equilibrium, kinetic, intra crystalline diffusion and thermodynamic studies. Iran. J. Chem. Chem. Eng. 2019, 38 (6): 63-81.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Souad Achour, Djamel Nibou, Samira Amokrane

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





