Toxicity and risk of induced second cancer for Hodgkin Lymphoma (HL) treatment using new modalities of radiotherapy for young patients
DOI:
https://doi.org/10.57056/ajet.v8i2.119Keywords:
TCP/NTCP, EAR, OED, Hodgkin Lymphoma, CancerAbstract
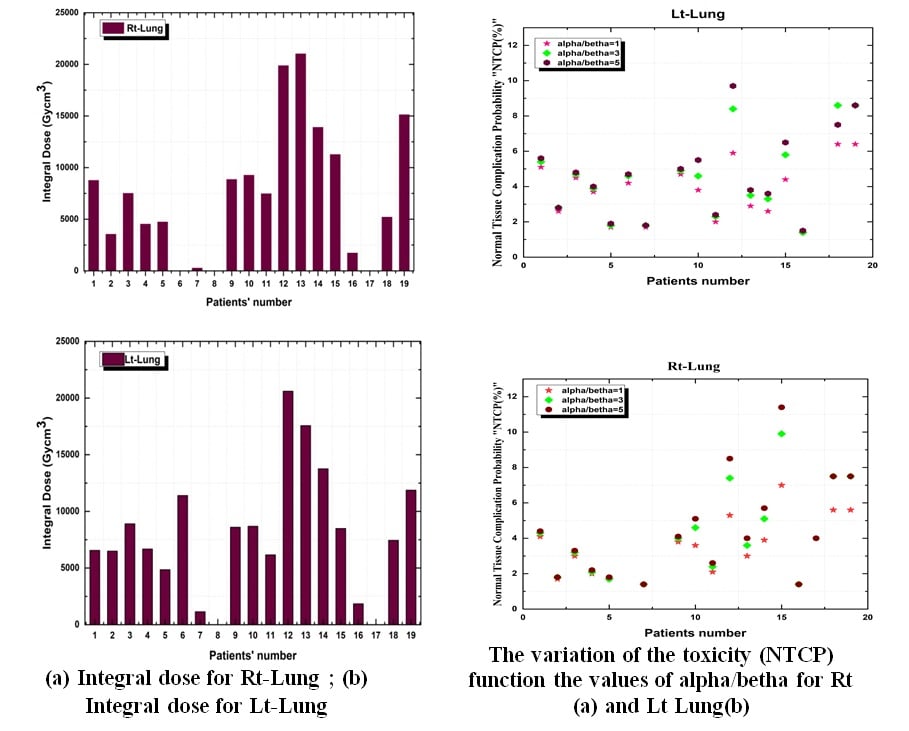
During the last decade new modalities of radiotherapy were implemented in different radiation oncology department in Algeria. Most of them are based on the concept of inverse modulated radiotherapy (IMRT), volumetric modulated radiotherapy (VMAT) and or helical tomotherapy (HT). The purpose of this study is to explore the trade-offs between cancer care, toxicities of organ’s at risk and the risk of induced second cancer in case of hodgkin lymphoma. A cohort of 20 young patients treatment plans using Field-in-Field radiotherapy (3D-FIF) were assessed using mathematical model to predict the toxicity calculated by the normal tissue complication probability (NTCP) using Lyman-Kutcher-Burman model and the estimated absolute risk (EAR) for the organ’s at risk in case of Rt and Lt lung. The associated induced second cancer risk was computed using the organ equivalent dose (OED) defined as a mechanistic model in the A Bomb survivors and hodgkin’s patients data relevant to radiotherapy. The results showed that the mean dose received at right and left lung are (7.81±4.6) Gy and (8.74±3.8) Gy respectively. The calculated NTCP for pneumonitis lung end point were 4.2% and 4.5% which correspond to EUD mean = (5.98±3.16) and (6.21±3.49) Gy. The associated estimated absolute risk of induced second cancer was obtained for Right and left lung and are 4.39±3.24 and 5.54±3.41 per 10000 P-Y. Higher risk was observed for three patients of the studied cohort. EAR and toxicity modelling is a better way to evaluate Hodgkin’s lymphoma specially when it comes of young patients where the risk of induced second cancer is more important.
References
R Number of cancer aroud the world registered in 2020 According to: Hodgkins lumphoma-Global cancer observatory. https://gco.iarc.fr/today/data/factsheets/cancers/33-HodgkinLymphoma-fact-sheet.pdf. Accessed March 2, 2022.
De Sanctis V, Bolzan C, D’Arienzo M, Bracci S, Fanelli A, Cox MC, Valeriani M, Osti MF, Minniti G, Chiacchiararelli L, Enrici RM. Intensity modulated radiotherapy in early stage Hodgkin lymphoma patients: Is it better than three dimensional conformal radiotherapy?. Radiation Oncology. 2012;7:1-9.
Weber DC, Johanson S, Peguret N, Cozzi L, Olsen DR. Predicted risk of radiation-induced cancers after involved field and involved node radiotherapy with or without intensity modulation for early-stage hodgkin lymphoma in female patients. International Journal of Radiation Oncology* Biology* Physics. 2011;81(2):490-497.
Niemierko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med phys. 1997;24(1):103-110.
Niemierko A. A generalized concept of equivalent uniform dose (EUD). Med Phys. 1999;26(6):1100.
kavanagh BD, Timmerman RD, Benedict SH et al. How should we describe the radiobiological effect of extracranial stereotaxic radiosurgery: equivalent uniform dose or tumour control probability? Med Phys. 2003; 30(3): 321-324.
Lyman JT. Normal tissue complication probabilities: variable dose per fraction. International Journal of Radiation Oncology* Biology* Physics. 1992;22(2):247-250.
Lyman JT. Complication probability as assessed from dose-volume histograms. Radiation Research. 1985;104(2s):S13-19.
Kutcher GJ, Burman C, Brewster L et al. Histogram reduction method for calculating complication probabilities for 3-dimensional treatment planning evaluations. Int J Rad Onc, Biol Phy. 1991; 21:137- 146.
de Crevoisier R, Fiorino C, Dubray B. Radiothérapie prostatique: prédiction de la toxicité tardive à partir des données dosimétriques. Cancer/Radiothérapie. 2010;14(6-7):460-468.
Dasu A. Toma-Dasu I. Olofsson J. Karlsson M. The use of risk estimation models for the induction of secondary cancers following radiotherapy. Acta Oncol. 2005; 44:339-347.
Thames HD. Hendry JH. Fractionation in radiotherapy. London-New
Terahara A, Niemierko A, Goitein M et al. Analysis of the relationship between tumour dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys. 1999; 45(2): 351-358;
Schneider U, Stipper A, Besserer J. Dose-response relationship for lung cancer induction at radiotherapy dose. Zeitschrift für Medizinische Physik. 2010;20(3):206-214.
Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. International Journal of Radiation Oncology* Biology* Physics. 1991;21(1):109-122.
Thieke C, Bortfeld T, Niemierko A, Nill S. from physical dose constraints to equivalent uniform dose constraints in inverse radiotherapy planning. Med Phys. 2003; 30(9): 2332-2339.
Schneider U. Mechanistic model of radiation-induced cancer after fractionated radiotherapy usin,g the linear quadratic formula. Med Phys. 2009, 36(4):1138-1143.
Schneider U, Sumila M, Robotka J, Gruber G, Mack A, Besserer J. Dose-response relationship for breast cancer induction at radiotherapy dose. Radiation oncology. 2011;6(1):1-7.
Schneider U, Stipper A, Besserer J. Dose-response relationship for lung cancer induction at radiotherapy dose. Zeitschrift für Medizinische Physik. 2010;20(3):206-214.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Assya Boughalia , Lyes Benfetima , Samia Saadi , Djemaa Bendjazia , Ouhid Boughrara

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





