Studies on the separation of Uranium from Algerian yellow cake using different processes: impurities determination
DOI:
https://doi.org/10.57056/ajet.v8i2.118Keywords:
yellow cake, uranium (VI), separation, extraction, XAD-7, Tri-n-butyl Phosphate, PrecipitationAbstract
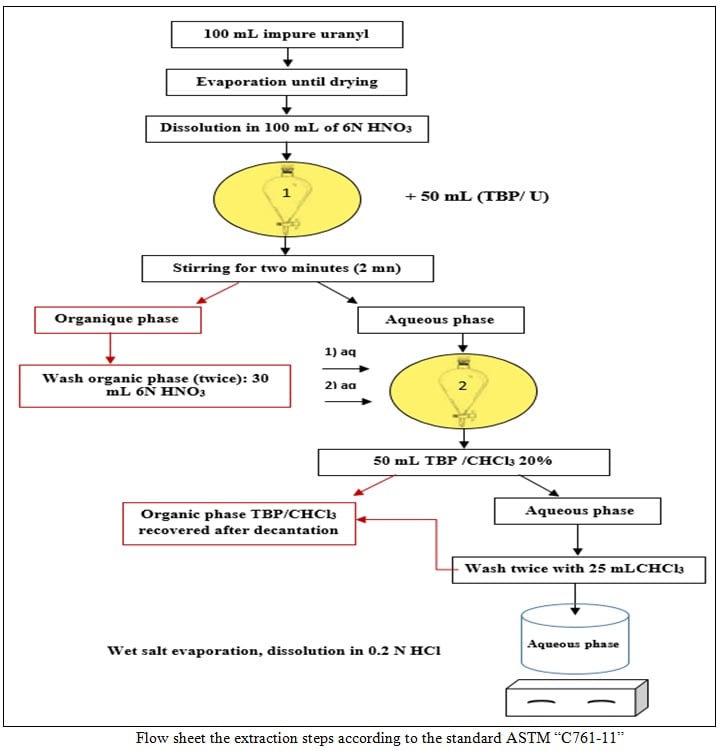
Analysis of impurities in uranium materials is crucial for quality control or the purity required by standards in the nuclear industry. This study involves the analysis of impurities in a sample of uranium ore concentrate produced from Tahaggart ore. The instrumental techniques used for the analysis are highly sensitive and susceptible to much interference following the uranium spectrum. Different processes for separating uranium from impurities for comparison were used to quantify them. The uranium sample is an Algerian yellow cake that was digested in nitric acid, separated by solvent extraction using the TBP/CHCl3 system, by extraction chromatography method using tributyl phosphate (TBP) impregnated on the Amberlite XAD-7 resin and by precipitation of uranium with hydrogen peroxide. The raffinate from each process is then analyzed by flame atomic absorption spectroscopy and flame photometry. The concentration of uranium is determined by the Potassium Bichromate method in concentrated solutions and by the Arsenazo III method in raffinates and eluates. Uranium extraction yields are 99.65% using TBP-CHCl3 and exceed 99% using TBP-impregnated XAD-7 resin. The results of the analysis of impurities in Algerian yellow cake after the separation of uranium using different processes show that the contents meet the ASTM C967-13 standard for the elements analyzed except for the iron element. A comparison of the results of the impurities analysis values in Algerian yellow cake by the three separation processes (liquid-liquid extraction (ASTM C761-11), extraction chromatography column, and precipitation) shows the absence of cadmium, titanium, lead, and chromium and the values of the manganese, zinc, and lithium are quite close. The analysis results for the solvent extraction and chromatographic column extraction processes showed that the values of magnesium, copper, and nickel are very close and identical for cobalt. The values of aluminium and iron are close.
References
de Souza AL, Cotrim ME, Pires MA. An overview of spectrometric techniques and sample preparation for the determination of impurities in uranium nuclear fuel grade. Microchemical Journal. 2013;106:194-201.
Manard BT, Bostick DA, Metzger SC, Ticknor BW, Zirakparvar NA, Rogers KT, Hexel CR. Rapid and automated separation of uranium ore concentrates for trace element analysis by inductively coupled plasma–optical emission spectroscopy/triple quadrupole mass spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy. 2021;179:106097.
Fletcher ND, Manard BT, Bostick DA, Bostick WD, Metzger SC, Ticknor BW, Rogers KT, Hexel CR. Determination of phosphorus and sulfur in uranium ore concentrates by triple quadrupole inductively coupled plasma mass spectrometry. Talanta. 2021;221:121573.
International Atomic Energy Agency, Advanced methods of process/quality control in nuclear reactor fuel manufacture, In IAEA, Proceedings of a Technical Committee meeting held in Lingen, Germany. 1999, 18-22.
Reis EL, Scapin M, Cotrim ME, Salvador VL, Pires MA. Determination of Nuclear fuel element components for the IEA-R1 Research Reactor by analytical Methods based on ED-XRF and ICP-OES. In International Nuclear Atlantic Conference 2009-INAC, 2009.
de Jesus Tavares M, e Barros JS, Matos F. Determination of uranium and impurities in Portuguese yellow-cakes. Talanta. 1977;24(5):326-328.
Satyanarayana K, Durani S. Separation and inductively coupled plasma optical emission spectrometric (ICP-OES) determination of trace impurities in nuclear grade uranium oxide. Journal of radioanalytical and nuclear chemistry. 2010;285(3):659-665.
Arruda MA. Trends in sample preparation, Nova Science Publishers, INC, New York: 2007.
Guerin N. Automated separation of actinides by extraction chromatography.Doctoral thesis, Laval University, Quebec, Canada, 2012.
Mahmoud J, Higginson M, Thompson P, Gilligan C, Livens F, Heath SL. Rapid separation of americium from complex matrices using solvent impregnated triazine extraction chromatography resins. Journal of Chromatography A. 2022;1669:462950.
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Essling AM, Graczyk D. Separation and preconcentration of uranium from acidic media by extraction chromatography. Analytica Chimica Acta. 1992;266(1):25-37.
Dwivedi VN, Mahanta PL, Premadas A. An integrated approach to the complete chemical analysis of magnesium or sodium diuranate (yellow cake) sample. Journal of Radioanalytical And Nuclear Chemistry. 2003;258(3):575-581.
C761-11, Standard Test Methods for Chemical, Mass Spectrometric, Spectrochemical, Nuclear and Radiochemical Analysis of Uranium Hexafluoride, ASTM West Conshohocken, 2018.
C1347-08, Standard Practice for Preparation and Dissolution of Uranium Materials for Analysis, ASTM West Conshohocken, 2008.
NF ISO 7097- 1, Nuclear fuel technology - Determination of uranium in solutions, uranium hexafluoride and solids - Part 1: titrimetric method by iron (II) reduction and potassium dichromate oxidation, 2006.
Fritz J, Johson-Richard JM, Colorimetric uranium determination with Arsenazo. Analytica Chimica. Acta. 1959, 20 : 164-171.
Savvin SB. Analytical use of arsenazo III: determination of thorium, zirconium, uranium and rare earth elements. Talanta. 1961;8(9):673-685.
Barros JS, de Jesus Tavares M, Afonso MH. Quality control of portuguese uranium concentrates. Inorganica Chimica Acta. 1984;94(6):309-312.
C967-13, Standard Specification for Uranium Ore Concentrate, ASTM West Conshohocken, 2013.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Sihem Khemaissia, Faiza Oudjer, Farida Semaoune, Asma Benturki, Fatiha Bendjeriou, Ramy Ahmed Khenich, Sara Bentafat, Amira Yousfi, Leila Mohelbi, Hayet Guettaf

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





