Sol-gel synthesis of ZnO nanoparticles for optmized photocatalytic degradation of Eriochrome Black T under UV irradiation
DOI:
https://doi.org/10.57056/ajet.v8i1.100Keywords:
Photocatalysis, ZnO, Box-benhken, UV-light, Eriochrome Black TAbstract
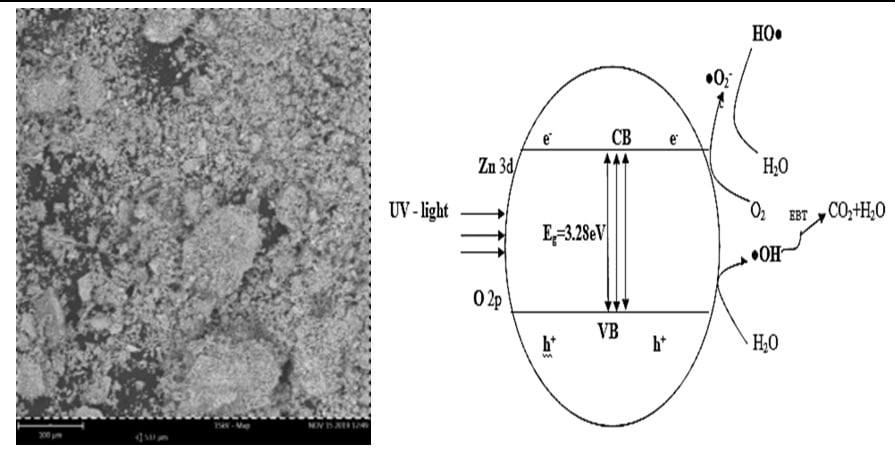
In this work, zinc oxide (ZnO) nanoparticles was synthesized by sol-gel method and characterized using x-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FT-IR), energy dispersive spectroscopy (EDS) and ultraviolet-visible (UV-Vis) spectrophotometry. The XRD analysis of the as synthesized catalyst revealed a hexagonal wurtzite structure. The average particle size and band gap values were 24.67nm and 3.28eV respectively. The peak observed at 452cm-1 corresponds to Zn-O stretching vibrational band. The effect of operating paramers such as initial concentration of eriochrome black-T (EBT), concentration of catalyst and pH of the solution was optimized using box-benhken design (BBD) and response surface methodology (RSM). The optimum photodegradation efficiency of 96.59% was obtained at 15.00mg of EBT concentration, 0.40g catalyst concentration and initial pH of 9.00. The degradation model was statistically remarkable with p < 0.0001% in which the EBT initial concentration and catalyst concentration were the most significant variables influencing the degradation of EBT over ZnO photocatalyst under UV irradiation.
References
Robinson T, Kian M, Basil N, Remediation of Dyes in Textiles Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative, Bioresources Technology. 2001; 77: 247-275.
Ezgi A, Mufit B, Mustafa Y, Removal Efficiency of a Calyx [4] Arene-Based Polymer for Water Solible Carcinogenic Direct Azo Dyes and Aromatic Amines. Journal of Hazard Material, 2008; 162 (2-3): 960-966.
Vaiano V, Matarangolo M, Sacco O, Sannino D, Photocatalytic Removal of Eriochrome Black T Dye over ZnO Nanoparticles doped with Pr, Ce or Eu. Journal of Chemical Engineering Transctions. 2017; 57: 625-630.
Wang J, Wang Z, Huang B, Ma Y, Liu X, Qin X, Zhang X, Dai Y, Photocatalytic Degradation of Eriochrom Black T Using ZnO Photocatalyst. Journal of Application Material Interface. 2012; 4: 4024-4030.
Sharma S, Abdullahi B, Ahmad A, Asar B, Photocatalytic Degradation of Eriochrome Back T Using Ammonium Phosphomolybdate Semicomductor as Catalyst Under Visible Light Irradiation .International Journal of Chemical Engineering. 2016; 42(1): 31-35.
Dunnil A, Van I, Priya A, Xia Q, Ozaki N, Photocatalytic Degradation of Eriochrome Black T Using C-doped TiO2 Photocatalyst Via Substitutional and Intestitial Dopig. Journal of Physical Chemistry. 2018; 37(1):123-129.
Bedoui A, Ahmadi MF, Bensalah N Gadri A, Comparative Study of Eriochrome Black T Treatment by BBD Anodic Oxidation and Fenton Process. Journal of Chemical Engineerig. 2009; 16:98-109.
Priya A, Benz X, Xu Q, Zhai Q, Photocatalytic Degradation of Eriochrome Black T Using Pure and N-doped ZnO Nanoparticles Prepared by Precipitation Method. International Journal of Applied Chemistry, RSC. 2015; 31(3): 121-124.
Aouni A, Fersi C, Cuartas -Uribe B, Bes-Pia A, Alcaina-Miranda M I, Dhabi M, Reactive Dyes Rejection and Tertile Effluent Treatment Study Using Ultrafiltration and Nanofiltration Processes. Desalination. 2012; 297:87-96.
Gaya U I, Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids. Springer. 2014; Dordrech: 2-14
Hamza A, Fatuase J T, Waziri S M, Ajayi O, Solar Photocatalytic Degradtion of Phenol Using ZnO . Journal of Chemical Engineering. 2013; 89-90.
Vaiano L, Sacco O, Sannino D, Ciambelli O, N-doped ZnO Nanoparticles Supported on ZnS Based Blue Phosphors in the Photocatalytic Removal of Eriochrome Black T. Journal of Engineering Transactions. 2016; 23: 232-237.
Xu X, Zhang Q P, Ong B T, Yuan H, Chen Y, Liu Y T, Xu M, Influence of Stabilzer on the Microstructrure and Photocatalytic Performance of ZnO Nanopowder Synthesized by Sol – gel Method. Journal of Nanotechnology. 2017; 50: 57-71.
Lu C,Wu Y, Mai F, Chung F, Wu Lin C, Chen C, Degradation Efficienties and Mechanism of ZnO Mediated Photocatalytic Degradation of Eriochrome Black T under Ultraviolet Light Irradiation. Journal of Molecular Catalysis. 2009; 30:159-165.
Raoufi D, Synthesis and Microstrucural Properties of ZnO Nanoparticles Prepared by Precipitation Method. Renewable Energy. 2013; 5(2): 932-937.
Savaranan V K, Gupta V, Narayan A, Comparative Study on Photocatalytic Activity of ZnO Prepared by Different Methods. Journal of Photochemistry and Photobiology. 2013; 181:133-141.
Sarith A, Shank H, Prakash T, Sampa C, Photocatalytic Degradation of Eriochrome Black T in Aqueous Medium under Ultraviolet and Visible Light Irradiation by TiO2 and ZnO Nanoparticles. Journal of Hazardous Material. 2017; 45(2): 335-340.
Supamas S, Schrank S, Josa P, Hiranok YT, Photocatalytic Degradation of Eriochrome Black T Using TiO2 as photocatalyst. Journal of Photochemistry and Photobiology. 2014; 34:225-258.
Maureen O O C, Nnaemeke OJ, Basil NA, Emeka EO, Photocatalytic Degradation of a Basic Dye using Zinc Oxide Nanocatalyst. International Letter of Chemistry, Physis and Astronomy. 2019; (81): 18-26.
Kian M, Lee S, Abdulhamid B, and China W, Multivariate Analysis of Photocatalytic-Mineralization of Eriochrome Black T Dye using ZnO catalyst under UV irradiation. Journal of Nanotechnology and Catalysis Research Centre. 2015; 42: 102-112.
Chen XW, Danden L, Zhen G, Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Research Letters, 2017; 12-13.
Yusuf A, Gaya U I, Mechanochemical Synthesis and Characterization of N-doped TiO2 for Photocatalytic Degradation of Caffeine. Journal of Nanochemistry.2018; 3(1): 29-35.
Yoshio K, Onodera A, H, Satoh A. H, Sakagami N, and Yamashita H, Crystal Structure of ZnO: Li At 293 K and 19 K by X-ray Diffraction. Ferroelectr. 2001; 264(1):133-138.
Dodoo-Arhin D, Asiedu T, Agyei-Tuffour B, Nyankson E, Obada D, Mwabora J. M, Photocatalytic Degradation of Rhodamine Dyes Using Zinc Oxide Nanoparticles. Material Today: Proceeding. 2021; 38(2): 809-815.
Algarni T. S, Abdah N. A. Y, Kahtani A. A and Aoussi, Photocatalytic Degradation of Some Dyes Under Solar Light Irradiation Using ZnO Nanoparticles Synthesized from Rosmarinus Officinalis Extract. Green Chemistry Letters and Reviews. 2022; 15(2): 460-473.
Kaman T and Selvakumar S. A.S, Biosynthesis of ZnO Nanoparticles Using Rambutan (Nephelium Lappaceum L) Peel Extract and their Photocatalytic Activity on Methyl Orange Dye. Journal of Molecular Structure. 2016; 1125: 358-365.
Jeevanandam J, Barhoun A, Chan Y. S, Dufresne A, Danguah M. K, Review on Nanoparticles and Nanostructures Materials: History, Sources, Toxicity and Regulations. Beilstein Journal of Nanotechnology. 2018; 9(1): 1050-1074.
Lanjwani M. F, Muhammad Y, Khuhawar T, Khuhawar J. Lanjwani A, Memon S. Q, Soomro W. A, Rind I. K, Photocatalytic Degradation of Eriochrome Black T Dye By ZnO Nanoparticles Using Multivariant Factorials, Kinetics and Isotherm Model. Journal of Cluster Science. 2022; 23: 293-8.
Fakhari S, Jamzad M, Fard H. K, Green Synthesis of Zinc Oxide Nanoparticles: a Comparison. Green Chemistry Letters and Reviews. 2019; 12(1): 19-24.
Shah S. N, Ali S. I, Ali S. R, Neem M, Bibi Y, Ali S. Raza S. M, Khan Y, and Sherwani S. K, Synthesis and Characterization of Zinc Oxide Nanoparticles for Antibacterial Applications. Journal of Basic and Applied Sciences. 2016; 12: 205-210.
Saravanann R, Shankar H, Rajasudha G, and Stephen A, Photocatalytic Degradation of Organic Dye By Nano ZnO. International Journal of Nanoscience. 2011; 10(1): 253-257.
Suresh P, Michael S, Nicholas N, Miguel G, Athanassion K, Mohammad HE, Patrick SM, Jeremy WJ, Anthony B, Kevin O, Dionysios DD, A Review on the Visible Light Active ZnO Photocatalysts for Environmental Applications. Chemosphere. 2012; 125:331-349.
Akhund A, Habibi-Yangjeh A, Ternaary Magnetic g-C3N4/Fe3O4/AgI Nanocomposites: Novel Recyclable Photocatalysts with Enhanced Activity in Degradation of Different Pollutants under Visible Light. Material Chemical Physics. 2016; 174: 59-69.
Shellofteh-Gohari M, Habibi-Yangjeh A, Novel Magnetically Separable Fe3O4@ZnO/AgCl Nanocomposites with Highly Enhanced Photocatalytic Activities under Visible Light Irradiation. Sep Purifi Technol. 2015; 147: 194-202.
Wang J, Jiang WJ, Liu D, Wei Z, Zhu YF, Photocatalytic Performance Enhanced via Surface Bismuth Vacancy of Bi6S2O15 Core/Shell Nanowires. Applied Catalysis of B Environment. 2015; 176: 306-314.
Huang N, Shu JX, Wang ZH, Chen M, Ren CG, Zhang W, One Step Pyrolytic Synthesis of ZnO Nanorods with Enhanced Photocatalytic Activity and High Photostability under Visible and UV Light Irradiation. Journal of Alloys Compounds. 2015; 648: 919-929.
Jurex A, Zheng X, Zhai Z, Zhao Q, Optimizaton for Photocatalytic Degradation of Eriochrome Black T Using Immobilized Ac/TiO2. Journal of Photochemistry and Photobiology, RSC. 2012; 31(2): 111-118.
Zhang J, Fu D, Xu Y, Liu C, Optimization of Parameters on Photocatalytic Degradation of Chloramphenicol Using TiO2 as Photocatalyst by Response Surface Methodology. Journal of Hazardous Environmental Science. 2010; 22: 1281-1289.
Zarei M, Niaei A, Salari D, Khataee A, Application of Response Surface Methodology for Optimization of Peroxi Coagulation of Textile Dye Solution Using Carbon Nanotube PTFE Cathode. Journal of Hazardous Materials. 2010; 173: 544-551.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Auwal Yusha’u, Muhammad Sulaiman Darma, Kamaluddeen Abubakar Isah

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





